Myocardial perfusion scintigraphy (MPS) is a non-invasive method that can be used to assess reversible left ventricular myocardial perfusion defect (<20% indicates limited and ≥20% indicates extensive ischaemia), and left ventricular ejection fraction (LVEF) at rest and at stress. Data on the utility of MPS used to stratify cardiac risk prior to abdominal aortic aneurysm (AAA) repairs are limited. We evaluated MPS as a stratification tool for patients scheduled for endovascular aneurysm repair (EVAR) or open repair, between 2013 and 2016 at Ashford and St Peter’s NHS Foundation Trust, and 4.9 years (median 2.8 years, interquartile range [IQR] 2.1–3.8) cardiovascular events (n=15, 17.9%) all-cause mortality (n=17, 22.6%). Of the 84 patients recruited (median age 75.7 years, IQR 69.4–79.6), 57 (67.9%) had limited and 27 (32.1%) extensive ischaemia, 62 (73.8%) underwent EVAR and 22 (26.2%) open repair. Compared with open repair patients, EVAR patients were older (median age 70.6 years vs. 76.4 years, p=0.015), had higher rates of extensive ischaemia (13.6% vs. 38.7%, p=0.025), and abnormal LVEF reserve (LVEF at stress minus LVEF at rest ≤0: 40.0% vs. 76.6%, p=0.011), while having lower rates of 30-day postoperative major adverse cardiac events (13.6% vs. 3.3%, p=0.040) but no difference for cardiovascular events (p=0.179) or 4.9 year all-cause mortality (22.7% vs. 22.6%, adjusted hazard ratio 0.80, 95% confidence interval [CI] 0.22 to 3.20, p=0.799). Our findings indicate that MPS provides valuable information for AAA repair procedure.
Introduction
Abdominal aortic aneurysm (AAA) is a progressive and potentially life-threatening condition, with a rupture risk of the order of 25% per annum for aneurysms exceeding 6 cm.1 Prophylactic AAA repair is, therefore, often necessary. Open repair has been the traditional method for many decades. However, since its inception in the early 1990s, endovascular aneurysm repair (EVAR) has increasingly been the procedure of choice,2 and has now overtaken open repair by a factor of between two and three to one.3,4
Prior to major vascular surgery, pre-operative assessment is routinely carried out to establish the patient’s risk profile to ensure that the patient is fit for surgery. This includes quantifying the patient’s health status, such as age, lifestyle factors and underlying comorbidities. Cardiac risk stratification is by far the most important factor in profiling the patient’s fitness for surgery. This is most commonly assessed by cardiopulmonary exercise testing, while myocardial perfusion scintigraphy (MPS) is less widely available. MPS detects coronary artery disease by measuring differences in perfusion, which reflect blood flow distribution through the left ventricular myocardium.5 Extensive ischaemia detected by MPS is associated with an increased risk of peri-operative and postoperative cardiac complications.6 The advantages of MPS over traditional methods lie in its ability to ‘directly’ detect myocardial ischaemia by an automated imaging system. MPS is suitable for individuals who are unable to perform physical exercise, such as patients with frailty or disability, which are mostly age-related, or those with severe effort-limiting claudication. This non-invasive method is suitable for those with coronary heart disease who are at high risk from coronary angiography,7 and those with asymptomatic coronary heart disease, and also provides information on left ventricular ejection fraction (LVEF).8 MPS is, therefore, a valuable alternative method for cardiac risk stratification and prognostication of outcomes. However, data on the role of MPS in the selection of a particular AAA repair procedure and postoperative outcomes, especially long-term cardiovascular events and mortality, are limited.
Herein, we conducted a study to evaluate the utility of MPS in AAA repair and prognostication of cardiovascular outcomes and mortality over 4.9 years.
Method
Patients, setting and study design
As part of a clinical audit, we retrospectively collected baseline information of 84 patients who were planned to undergo elective AAA repair between March 2013 and March 2016, at Ashford and St. Peter’s NHS Foundation Trust. Pre-operative and peri-operative data were collected from medical notes, including demographic factors (age and sex), American Society of Anesthesiologists (ASA) physical status classification, history of smoking within the past year, medications, pre-existing comorbidities including obesity (body mass index [BMI] ≥30 kg/m2), diabetes, atrial fibrillation, acute coronary syndrome (ACS), congestive heart failure (CHF), transient ischaemic attack, stroke, chronic obstructive pulmonary disease, chronic kidney disease and previous cardiac intervention. Follow-up information on 30-day postoperative major adverse cardiac events (MACE), comprising acute myocardial infarcts and cardiac-related deaths, was recorded. Additionally, data on cardiovascular events (defined as hospitalisation for coronary disease, CHF, stroke, or peripheral arterial disease) and all-cause mortality were obtained up to the end point of our study (maximum 4.9 years, median 2.8 years, interquartile range [IQR] 2.1–3.8). Radiological databases were used to obtain the size of the AAAs and MPS results. We contacted primary care teams, where necessary, to complete any missing information.
Myocardial perfusion scintigraphy
Before proceeding to either EVAR or open AAA repair, patients underwent cardiac risk stratification by MPS, which was conducted by a radionuclide radiologist. The MPS studies were performed with a gated stress/gated rest 99mTc-tetrofosmin protocol, beginning with the stress study, in which the patient was injected with the tracer 99mTc-tetrofosmin at a dose between 600 and 1,000 MBq prior to performing maximal exercise on an ergometer for at least one minute, after the slow intravenous injection of the pharmacological stress, adenosine vasodilator regadenoson (400 µg), for at least two minutes after the injection of the tracer. The rest study with injection of 99mTc-tetrofosmin (600–1,000 MBq) was then performed in the afternoon or the next day. Images were obtained using dual-head single-photon emission computed tomography (SPECT) cameras (Siemens Symbia S, Erlangen, Germany) and the non-gated and gated images were reviewed on the QGS/QPS software (Cedars-Sinai Medical Center Cardiac Suite, Los Angeles, US) for mapping of reversible perfusion defects; scintigraphic evidence of ischaemia was considered when the defect size was ≥20%. Since fixed ischaemia has been shown to have no prognostic value, only the degree of reversibility of ischaemia is used for cardiac risk stratification.9 MPS also measures LVEF at rest and at stress, which were used to relate to outcomes in the present study.
Surgical procedure
Surgery was performed under general or spinal anaesthesia by a vascular surgeon. Open repair was performed with an abdominal incision to allow access for placing the vascular stent graft. Endovascular repair involved the transluminal insertion of an expandable stent graft system through the femoral arteries into the aneurysmal region of the aorta and iliac arteries.
Selection of surgical procedure was conducted by a multi-disciplinary team (MDT), including vascular surgeons, radionuclide radiologist, vascular radiologist and anaesthetist. Full discussion involving patient’s personal choice and patient’s best interests were taken into account in the decision-making process. EVAR was generally considered for patients who were older than 70 years, favourable vascular anatomy and site of aneurysms (infrarenal), had evidence of extensive ischaemia and poor fitness for surgery. Open repair was considered for patients who were younger, because of longer graft durability, and those not suitable for EVAR, such as challenging anatomy, including tortuous arteries and juxtarenal aneurysms not amenable to fenestrated or other complex aneurysm devices. We followed published guidelines on AAA repair, which recommend intervention eight weeks from time of referral for men with AAA ≥5.5 cm who are fit for surgery and not declining.10 The threshold for AAA repair is generally lower in women at about 5 cm.10,11 The rate of growth of the aneurysm (expansion of ≥1 cm/year) or extensive ischaemia were additional factors for consideration of surgical repair in those with smaller size of AAA.12
Categorisation of variables
Data for underlying comorbidities were dichotomised according the absence or presence of the condition. Age of patients and size of AAA were grouped at their respective median values and obesity was defined by a BMI ≥30 kg/m2. Limited ischaemia was considered for those with MPS defect size <20% and extensive ischaemia for those with MPS defect size ≥20%.9 LVEF ≤45% was considered to be poor, while LVEF reserve (ΔLVEF) was calculated as LVEF at stress minus LVEF at rest; ΔLVEF ≤0 was considered as abnormal.13
Statistical analysis
Chi-squared test was used to assess group differences between patients with limited ischaemia and those with extensive ischaemia, poor LVEF and the choice of vascular surgery procedure and outcomes including 30-day postoperative MACE, rates of cardiovascular events and mortality. The non-parametric Mann-Whitney test was used to assess group differences among continuous variables. Multiple logistic regression was used to assess the likelihood of selecting surgical procedure (dependent variable) according to reversibility of ischaemia based on MPS (independent variable) and Cox regression to compare the risk of mortality between EVAR and open repair over 4.9 years. Two models were conducted, unadjusted and adjusted for age, sex, smoking within the past year, size of AAA and comorbidities. Survival probabilities up to the end point after AAA repair by the two surgical procedures were calculated using Kaplan-Meier plot and log-rank test.
Results
There were 72 male (median age 77.4 years, IQR 67.9–79.6) and 12 female patients (median age 77.1 years, IQR 75.7–82.3), 57 (67.9%) had limited and 27 (32.1%) extensive ischaemia, 22 (26.2%) received open surgery and 62 (73.8%) underwent EVAR. There were no age differences between men and women (p=0.068) or between limited and extensive ischaemic groups (p=0.222). The majority of patients were fit for surgery with ASA grade 3 in 97.5% and grade 4 in 2.5% of the patients, and, similarly, 95.1% of patients were functionally independent while 4.9% were partially dependent (needing some help with their care).
Compared with patients who underwent open AAA repair, patients who underwent EVAR were significantly older (median age 76.4 years, IQR 71.4–80.1 vs. 70.6 years IQR 65.4–77.4, p=0.015) and a higher proportion had extensive ischaemia (38.7% vs. 13.6%, p=0.025) (table 1).
![MacGregor Table 1. Characteristics of 72 male (median age 77.4 years, interquartile range [IQR] 67.9–79.6) and 12 female patients (median age 77.1 years, IQR 75.7–82.3) who underwent endovascular aortic repairs (EVAR) or open abdominal aortic aneurysm (AAA) surgery](https://bjcardio.co.uk/wp-content/uploads/2018/07/MacGregor-Table-1.png)
There were six (7.1%) cases with 30-day postoperative MACE, 15 (17.9%) cardiovascular events and 17 (22.6%) all-cause mortality over 4.9 years. The extent of ischaemia, LVEF at rest or LVEF reserve assessed by MPS, and pre-existing conditions, including cardiac intervention, ACS, CHF and symptoms of exertional dyspnoea, did not relate significantly to either 30-day postoperative MACE, cardiovascular events or mortality, except for a significantly higher rate of mortality among those with exertional dyspnoea compared with those without the symptom (41.2% vs. 15.6%, χ2=5.3, p=0.029) (table 2).

The rates of 30-day postoperative MACE were significantly lower for EVAR than those with open repair (3.3% vs. 18.2%, p=0.040, adjusted odds ratio 0.15, 95% confidence interval [CI] 0.03 to 0.90, p=0.044). The maximum time since AAA repair to the end of the study was 4.9 years (median 2.83 years, IQR 2.1–3.8). There were no differences in cardiovascular events between EVAR and open repair (21.0% vs. 9.1%, p=0.179). Kaplan-Meier survival analysis shows that there were no significant differences in all-cause mortality between EVAR and open repair over the course of 4.9 years postoperation (22.6% vs. 22.7%, log-rank test χ2=0.04, p=0.837) (figure 1). Cox regression, adjusted for age, sex, smoking and comorbidities, shows the hazard ratio for having all-cause mortality over 4.9 years from EVAR compared with open repair was 0.80 (95% CI 0.22 to 3.20, p=0.799).

Among 26 patients with evidence of extensive ischaemia detected by MPS, 22 (84.6%) were treated with an antiplatelet and 21 (87.5%) with a 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductase inhibitor (statin), 18 (72.0%) of whom were treated with both, while none of the patients were untreated with either of these agents. Of the 15 patients with pre-existing ACS, nine (60%) were treated with an antiplatelet, 11 (73.3%) with a statin, among whom seven (50.0%) were treated with both, while one patient was untreated with either agents. Among 21 patients who previously underwent percutaneous coronary intervention, 15 (71.4%) were treated with an antiplatelet, 19 (90.1%) with a statin, among whom 13 (61.9%) were treated with both, while none of the patients was untreated with either agents (table 3).
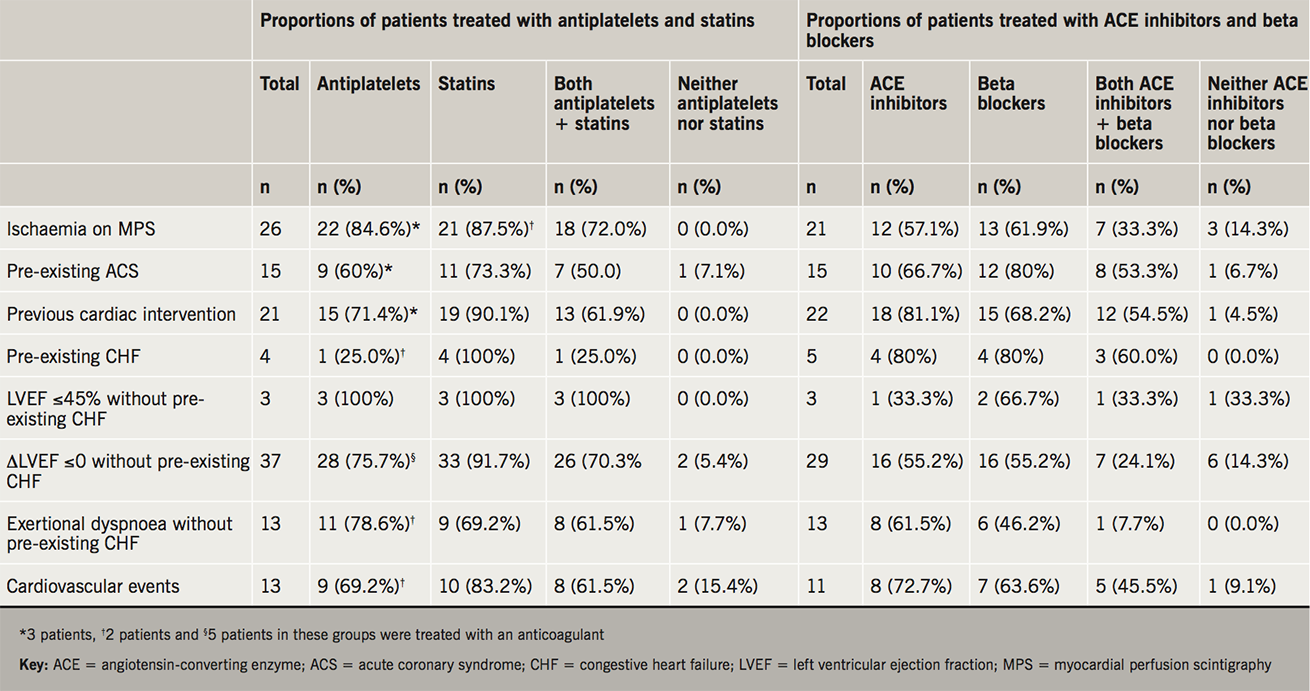
Of the five patients with pre-existing CHF, four (80%) were treated with an angiotensin-converting enzyme (ACE) inhibitor, and four (80%) with a beta blocker, three (60%) of whom were treated with both, while none were untreated with either of these drugs. Among three patients with LVEF ≤45% detected by MPS without pre-existing CHF, one was treated with an antiplatelet and two with a statin, one of whom was treated with both, while one was untreated with either drugs. There were 13 patients with symptoms of exertional dyspnoea without pre-existing CHF, eight (61.5%) of them were treated with an antiplatelet, six (42.6%) with a beta blocker, among whom one was treated with both, while none were untreated with these agents (table 3).
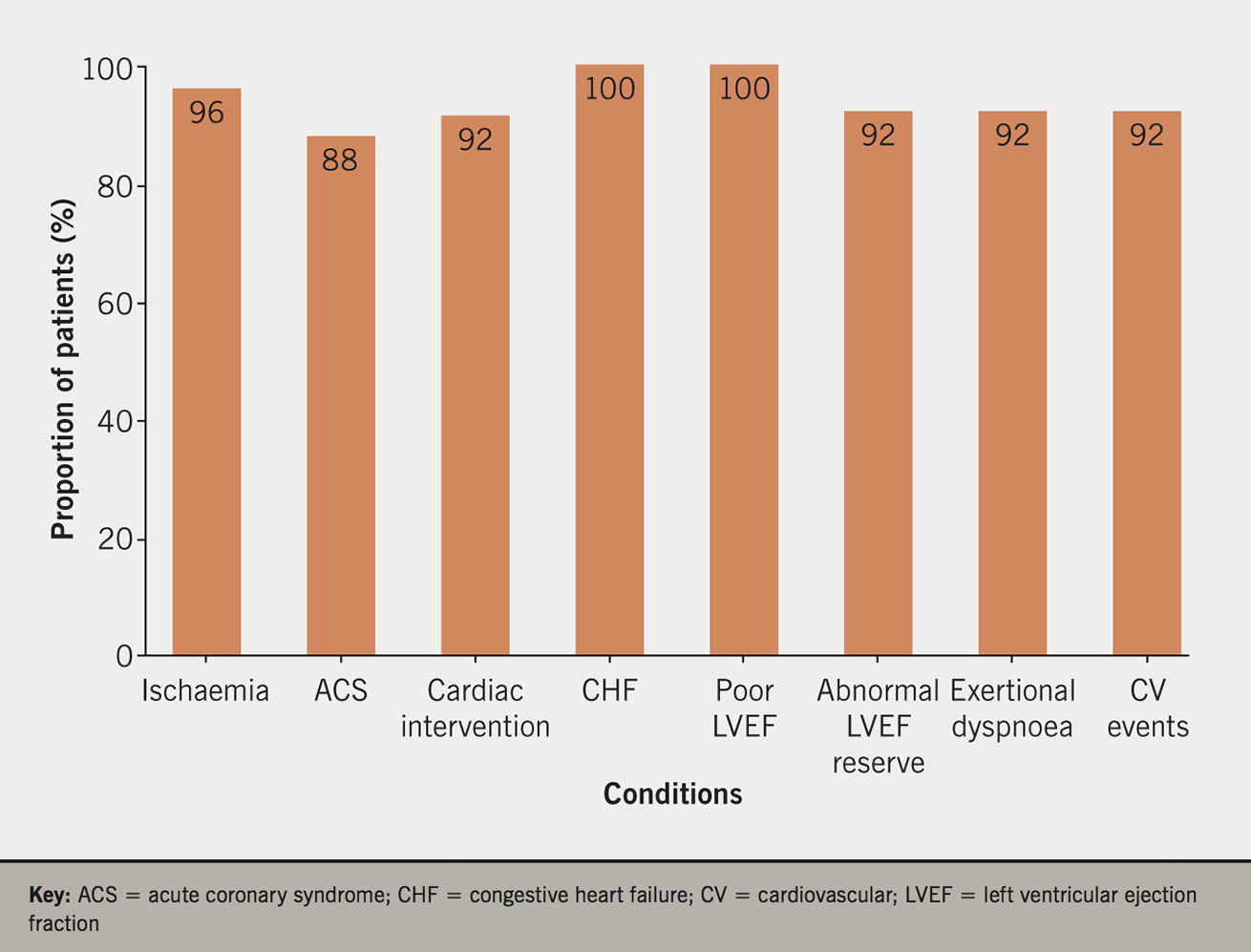
We also observed that the majority of patients with ischaemia, pre-existing ACS and cardiac intervention were treated with ACE inhibitors and beta blockers, and, similarly, the majority of patients with pre-existing CHF, those with LVEF ≤45% or those with symptoms of exertional dyspnoea, were treated with antiplatelets and statins (table 3). The majority of patients with evidence of ischaemia or CHF were treated with at least one of these four medications (figure 2).
Discussion
In the present study, we demonstrate that when using MPS to stratify cardiac risk prior to AAA repair procedure, EVAR outperformed open repair in short-term postoperative MACE and had similar long-term cardiovascular events and mortality rates, despite the patients undergoing EVAR being significantly older and at greater cardiac risk (extensive ischaemia indicated by MPS defect size ≥20%). Our findings are consistent with most previous studies,14,15 with the exception of a few studies, including that by Greenhalgh et al.,16 showing no early benefit from EVAR compared with open repair; the authors suggested that this was due to a higher proportion of high-risk patients receiving EVAR. Our findings are robust since most of the major comorbidities that could potentially influence the outcomes were taken into account by multiple logistic and Cox regressions, thus, allowing us to independently focus on the utility of MPS in assigning the most suitable AAA repair procedures to minimise postoperative MACE in the short term and mortality in the long term.
EVAR has now emerged as the main procedure for AAA repair. The figure of 74.4% of patients undergoing EVAR in our study is consistent with the published figure of 78% in the US.4 Our observation of older patients undergoing EVAR than those who undergo open repair is in line with previous studies.2 A number of reasons explain this age difference between procedures, including the greater risk of open surgery in older patients, while younger patients are more likely to choose open repair because of the greater durability of the graft, although the quality of EVAR stent grafts is improving.17 Older age is associated with greater risk of chronic conditions and frailty, rendering patients unsuitable for major open surgery. Our study found no statistical differences in age between limited and extensive ischaemia groups of patients. It should be borne in mind that the individual patient’s choice, after comprehensive explanation of treatment benefits and risks, plays an important role in the selection of a particular procedure during the shared decision-making process.
Our present study found that other than older age, MPS was the only factor to significantly influence the selection of EVAR or open surgery, independent from a number of underlying comorbidities and age of the patients. It has become evident that MPS provides valuable information for assigning patients to the appropriate AAA repair procedure as indicated by 30-day operative and long-term outcomes, which is in line with a number of previous studies.14,15,18 Etchells et al. performed a meta-analysis of nine studies (n=1,179 patients) to investigate the prognostic value of MPS for peri-operative cardiac complications in patients undergoing non-cardiac vascular surgery and found that limited ischaemia (<20% of the myocardial segments) did not associate with the risk of complications, while there were incremental increases in complications with the extent of reversible ischaemia ≥20%, indicated by likelihood ratios: 1.6 (95% CI 1.0 to 2.6) for 20–29% reversibility, 2.9 (95% CI 1.6 to 5.1) for 30–39% reversibility, 2.9 (95% CI 1.4 to 6.2) for 40–49% reversibility and 11 (95% CI 5.8 to 20) for ≥50% reversibility.9 Although there is a definite relationship between the degree of myocardial ischaemia and prognosis, it remains unclear that prophylactic revascularisation solely to prevent ischaemia at the time of surgery would improve outcomes.19
The increasing popularity of EVAR is due to a number of favourable factors, including its reduced invasiveness, shorter time to recovery and better early outcomes,14,15,18 but it is generally not the procedure of choice for younger patients due to shorter graft durability, and the requirement for frequent computerised tomography (CT) scanning to monitor leakage. On the other hand, open surgical repair requires general anaesthesia for laparotomy and there is frequent need for aortic cross-clamping lasting at least 30 minutes,20 but it has its own advantages, including longer graft durability, which is beneficial for younger patients and when the anatomy of the aneurysm is not suitable for EVAR placement.15,21,22 Our mortality data show no differences in mortality rates over 4.9 years between the two surgical procedures, but after this period there may be a divergence, since patients who underwent EVAR were older by about six years.
Aneurysms arising close to or above the kidneys are more challenging by EVAR; patients who had suprarenal or juxtarenal aneurysms are considered for open repair if there is no fenestrated graft alternative.23 In our study, there was one patient (1.2%) with suprarenal aneurysm who was treated with open repair.
Patients undergoing AAA repair, as in our cohort, have high risk of cardiovascular events. We treated our patients according to published guidelines with an antiplatelet and lipid-lowering medication (statin) prior to AAA repair. Patients with CHF were managed by cardiologists according to National Institute for Health and Care Excellence (NICE) guidelines,24 including beta blockers and ACE inhibitor (or angiotensin-receptor blocker if there are intolerable side effects) and were considered for further investigation and revascularisation, if appropriate, after review of MPS findings. The issue of commencing beta blockers in asymptomatic patients prior to surgery has been particularly contentious in recent years. Nevertheless, current guidelines would recommend considering these medications in those with demonstrable ischaemia.25 In the present study, virtually all patients with evidence of extensive ischaemia, pre-existing diagnosed CHF and those with clinical features of CHF, including exertional dyspnoea or poor LVEF by MPS without pre-existing diagnosis of CHF, were treated with beta blockers, as well as ACE inhibitors. Evidence from our study of no increase in cardiovascular events in this high-risk group of patients reinforces the value of intensive drug therapy for such patients.
Strengths and limitations
The strengths of the present study lie in its completeness of comorbidities, which provide the robust interpretation of the influences of MPS on the selection of surgical procedures and the outcomes from these procedures, with a relatively long follow-up interval. The study is limited by its retrospective nature and a relatively small sample, therefore, bias might have been introduced which affects our findings, particularly the early outcomes where few events occurred. Bias on selection of one or the other procedure may also exist and vary between units depending on facilities and expertise. Reversibility of ischaemia cut-off at 20% is based on previous work, but this figure is somewhat arbitrary as severity of ischaemia varies with cut-off values;9 the higher the cut-off level, the more extensive is ischaemia and peri-operative complications, while less patients are included. Future perspectives are suggested, including establishing a ‘risk score calculator’ based on MPS, age, sex and comorbidities of the patients to guide the most suitable procedure for AAA repair.
Conclusion
A part of clinical audit, we have demonstrated that MPS is a valuable tool for stratifying cardiac risk in patients undergoing AAA repair in a district general hospital vascular hub. MPS provides a wealth of information, including a guide to choice of AAA repair technique and assessment of medical treatment based on the ability of MPS to measure the extent of cardiac ischaemia and LVEF, both at rest and at stress. To complete this audit, we are planning to conduct a prospective registry study of a large cohort of patients across different centres with complete data collection and longer-term outcomes, including cardiovascular events and mortality. We plan to have primary care teams to update outcomes every month.
In conclusion, our findings indicate that MPS, used for cardiac risk stratification, provides valuable guidance for assigning patients for the most suitable AAA repair procedure.
Key messages
- Since its inception in the early 1990s, endovascular aneurysm repair (EVAR) has increasingly been the procedure of choice for abdominal aortic aneurysm (AAA) repair, and has now overtaken open repair by a factor of between two and three to one
- Prior to major vascular surgery, pre-operative assessment is routinely carried out to establish the patient’s risk profile and to ensure that the patient is fit for surgery. This is most commonly assessed by cardiopulmonary exercise testing while myocardial perfusion scintigraphy (MPS), a method used to assess the extent of reversible and fixed cardiac ischaemic defects, is less widely available
- In this study, we used MPS to stratify cardiac risk to assist the selection of patients to either EVAR or open AAA repair
- Nearly all patients with evidence of extensive ischaemia and congestive heart failure were treated with either an antiplatelet, lipid-lowering drug, ACE inhibitor or beta blocker
- EVAR had similar long-term cardiovascular events and all-cause mortality rates despite the patients undergoing EVAR being significantly older and having greater cardiac risk indicated by MPS
- We have demonstrated that MPS provides valuable information for AAA repair procedure
Acknowledgements
We are grateful to Mr Paul Thomas (Consultant Surgeon, Epsom and St. Helier University Hospitals) and Dr Shirish Prabhudesai (Consultant Vascular Radiologist, Ashford and St. Peter’s Foundation Trust) for helpful comments.
Conflict of interest
None declared.
References
1. UK Small Aneurysm Trial Participants. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. Ann Surg 1999;230:289–97. https://doi.org/10.1097/00000658-199909000-00002
2. Schwarze ML, Shen Y, Hemmerich J, Dale W. Age-related trends in utilization and outcome of open and endovascular repair for abdominal aortic aneurysm in the United States, 2001–2006. J Vasc Surg 2009;50:722–9. https://doi.org/10.1016/j.jvs.2009.05.010
3. Waton S, Johal A, Heikkila K, Cromwell D, Boyle J, Loftus I. National vascular registry: 2017 annual report. London: The Royal College of Surgeons of England, November 2017. Available from: https://www.vsqip.org.uk/reports/2017-annual-report/
4. Dua A. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg 2014;59:1512–17. https://doi.org/10.1016/j.jvs.2014.01.007
5. Dvorak RA, Brown RK, Corbett JR. Interpretation of SPECT/CT myocardial perfusion images: common artifacts and quality control techniques. Radiographics 2011;31:2041–57. https://doi.org/10.1148/rg.317115090
6. Leppo J, Plaja J, Gionet M, Tumolo J, Paraskos JA, Cutler BS. Noninvasive evaluation of cardiac risk before elective vascular surgery. J Am Coll Cardiol 1987;9:269–76. https://doi.org/10.1016/S0735-1097(87)80374-1
7. Greenwood JP, Ripley DP, Berry C et al.; CE-MARC 2 Investigators. Effect of care guided by cardiovascular magnetic resonance, myocardial perfusion scintigraphy, or NICE guidelines on subsequent unnecessary angiography rates: the CE-MARC 2 randomized clinical trial. JAMA 2016;316:1051–60. https://doi.org/10.1001/jama.2016.12680
8. Bax JJ, Bonow RO, Tschöpe D, Inzucchi SE, Barrett E, Global Dialogue Group for the Evaluation of Cardiovascular Risk in Patients With Diabetes. The potential of myocardial perfusion scintigraphy for risk stratification of asymptomatic patients with type 2 diabetes. J Am Coll Cardiol 2006;48:754–60. https://doi.org/10.1016/j.jacc.2006.04.077
9. Etchells E, Meade M, Tomlinson G, Cook D. Semiquantitative dipyridamole myocardial stress perfusion imaging for cardiac risk assessment before noncardiac vascular surgery: a meta-analysis. J Vasc Surg 2002;36:534–40. https://doi.org/10.1067/mva.2002.126563
10. Public Health England. Abdominal aortic aneurysm screening across the UK. Available at: https://phescreening.blog.gov.uk/2017/01/20/abdominal-aortic-aneurysm-screening-across-the-uk/
11. Ferket BS, Grootenboer N, Colkesen EB et al. Systematic review of guidelines on abdominal aortic aneurysm screening. J Vasc Surg 2012;55:1296–304. https://doi.org/10.1016/j.jvs.2010.10.118
12. The Vascular Society for Great Britain and Ireland. Abdominal aortic aneurysm (AAA). Available at: https://www.vascularsociety.org.uk/patients/conditions/2/abdominal_aortic_aneurysm_aaa
13. Tamarappoo BK, Fong Ling L, Cerqueira M, Hachamovitch R. Independent prognostic value of left ventricular contractile reserve and chronotropic response in patients with reduced left ventricular ejection fraction undergoing vasodilator stress myocardial perfusion imaging with Rb-82 positron emission tomography. Eur Heart J Cardiovasc Imaging 2018;19:442–9. https://doi.org/10.1093/ehjci/jex157
14. Lederle FA, Freischlag JA, Kyriakides TC et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA 2009;302:1535–42. https://doi.org/10.1001/jama.2009.1426
15. United Kingdom EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1863–71. https://doi.org/10.1056/NEJMoa0909305
16. Greenhalgh RM, Brown LC, Epstein D, Kwong G, Powell JT, Sculpher MJ. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet 2005;365:2187–92. https://doi.org/10.1016/S0140-6736(05)66628-7
17. Maudet A, Daoudal A, Cardon A et al. Endovascular treatment of infrarenal aneurysms: comparison of the results of second-and third-generation stent grafts. Ann Vasc Surg 2016;34:95–105. https://doi.org/10.1016/j.avsg.2015.12.020
18. Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG; EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 2004;364:843–8. https://doi.org/10.1016/S0140-6736(04)16979-1
19. Young EL, Karthikesalingam A, Huddart S et al. A systematic review of the role of cardiopulmonary exercise testing in vascular surgery. Eur J Vasc Endovasc Surg 2012;44:64–71. https://doi.org/10.1016/j.ejvs.2012.03.022
20. Attia RR, Murphy JD, Snider M, Lappas DG, Darling RC, Lowenstein E. Myocardial ischemia due to infrarenal aortic cross-clamping during aortic surgery in patients with severe coronary artery disease. Circulation 1976;53:961–5. https://doi.org/10.1161/01.CIR.53.6.961
21. Sicard GA, Zwolak RM, Sidawy AN, White RA, Siami FS; Society for Vascular Surgery Outcomes Committee. Endovascular abdominal aortic aneurysm repair: long-term outcome measures in patients at high-risk for open surgery. J Vasc Surg 2006;44:229–36. https://doi.org/10.1016/j.jvs.2006.04.034
22. Becquemin JP, Pillet JC, Lescalie F et al. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low-to moderate-risk patients. J Vasc Surg 2011;53:1167–73. https://doi.org/10.1016/j.jvs.2010.10.124
23. Sugimoto M, Takahashi N, Niimi K, Kodama A, Banno H, Komori K. Long-term fate of renal function after open surgery for juxtarenal and pararenal aortic aneurysm. J Vasc Surg 2018;67:1042–50. https://doi.org/10.1016/j.jvs.2017.07.121
24. National Institute for Health and Care Excellence. Acute heart failure: diagnosis and management. London: NICE, 2014. Available from: https://www.nice.org.uk/guidance/cg187/chapter/1-Recommendations
25. Kristensen SD, Knuuti J, Saraste A et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014;35:2383–431. https://doi.org/10.1093/eurheartj/ehu282