Introduction
Venous thromboembolism (VTE) is a common source of morbidity and mortality: for example, the annual incidence of deep vein thrombosis (DVT) and pulmonary embolism (PE) in the UK have been estimated as 1/10,000 and 3–4/10,000 individuals, respectively, although the latter figure is considered to be an underestimate.1,2 The majority of cases of VTE occur during or following hospitalisation, and VTE has been described as the primary source of avoidable mortality in hospital;3 accordingly, more than 95% of such patients received a VTE risk assessment in 2016.4
Vitamin K antagonists (VKA), the standard of care for managing VTE for decades, are effective at reducing the risk of recurrence or stroke, but these drugs have a narrow therapeutic range, requiring frequent blood tests and adjustment of the dose.5 Warfarin, the most commonly used VKA, has pharmacodynamic and/or pharmacokinetic interactions with a wide range of medicines and other ingested substances that can increase the risk of recurrence of VTE, or of bleeding.6 The therapeutic class of non-VKA oral anticoagulants (NOACs) are as effective as warfarin for optimising outcomes, with a lower burden of treatment, and their therapeutic use in patients with VTE has increased in recent years.7 Edoxaban is a NOAC that acts by inhibition of clotting Factor Xa. This article summarises clinical outcomes from the Hokusai-VTE study,8 a randomised comparison of edoxaban versus warfarin, in patients with VTE.
The Hokusai-VTE study
Objectives and design
The objective of the Hokusai-VTE study was to establish non-inferiority of the Factor Xa inhibitor, edoxaban, compared with the VKA, warfarin, which represented the standard of care at the time of the study.8 The trial population were adults (≥18 years) with acute, symptomatic DVT in the popliteal, femoral, or iliac veins or acute, symptomatic PE, with or without concomitant DVT. Exclusion criteria included prior receipt of more than one dose of a VKA, aspirin treatment at a dose of >100 mg or conditions likely to require use of warfarin or low-molecular weight heparin (LMWH). A total of 8,240 patients (4,921 with DVT and 3,319 with PE) were randomly allocated to treatment with once-daily edoxaban 60 mg or warfarin, with stratification for presence of DVT or PE and dose of edoxaban (see below).
Patients received initial treatment with enoxaparin or unfractionated heparin for at least five days. Warfarin was given concurrently with heparin, and edoxaban was given after discontinuation of heparin, each for at least three months and not more than 12 months, based on local clinical practice. The dose of edoxaban was reduced to 30 mg once daily, for patients with weight ≤60 kg, creatinine clearance 30–50 ml/min, or receipt of potent P-glycoprotein inhibitors. Treatment blinding was maintained using a double-dummy design, and performance of ‘sham’ international normalised ratio (INR) measurements in the edoxaban group, at the same (monthly) intervals as used for patients receiving warfarin.
The primary study outcome was the incidence of symptomatic recurrent VTE, defined as a DVT or fatal or non-fatal PE. Non-inferiority was achieved if the upper 95% confidence interval (CI) for the hazard ratio (HR) for the primary outcome for edoxaban versus warfarin did not exceed 1.5. Key secondary efficacy outcomes were this composite plus either all-cause death or cardiovascular death. The main safety outcome was the incidence of clinically relevant bleeding, defined as major or clinically relevant non-major (CRNM) bleeding. The primary end point and deaths related to PE were adjudicated; causality of other cardiovascular and bleeding events were defined on the basis of available objective evidence.
Outcomes in the Hokusai-VTE study
Main efficacy and safety outcomes
The rate of recurrent VTE on edoxaban achieved non-inferiority with warfarin during both the overall study period and the on-treatment period (p<0.001 for both, figure 1). There were no significant differences in outcomes between treatments for patients with DVT or PE at baseline. The main safety outcome, major or CRNM bleeding, was reduced significantly in the edoxaban group versus the warfarin group, driven mainly by a reduction in CRNM bleeding (figure 1). The HR for any bleeding was also reduced in favour of edoxaban.
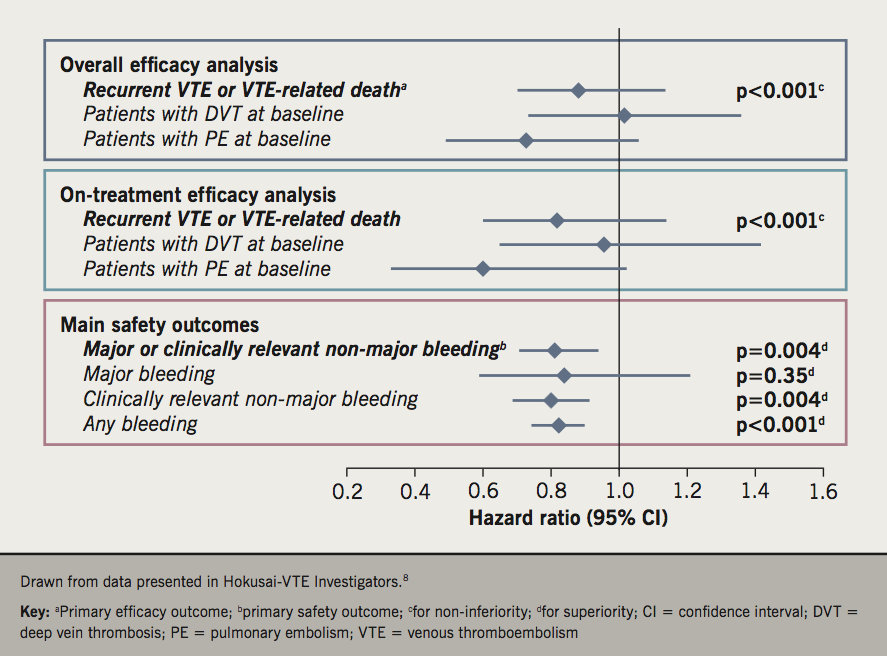
Efficacy and safety outcomes were comparable between the overall population and patients who received edoxaban 30 mg (figure 1). The efficacy and safety of edoxaban versus warfarin was generally similar across a broad range of other pre-specified subgroups. There were no significant interactions between efficacy outcomes and demographic, disease or treatment characteristics. Subgroup analyses suggested a marginally greater reduction in the risk of the main safety outcome for edoxaban versus warfarin in patients <60 versus ≥60 years of age (p for interaction [pint] =0.0175), and in Asian patients versus other ethnicities (pint=0.0266), which are unlikely to be of clinical significance.
Results in patients with right ventricular dysfunction
The main publication of Hokusai-VTE included an analysis in patients with or without right ventricular dysfunction, defined here as an N-terminal pro-B-type natriuretic protein (NT-proBNP) level of ≥500 pg/ml (present in 28% of the 3,319 patients with PE at baseline).8 Additionally, right ventricular dysfunction was diagnosed in a treatment-blinded manner on the basis of a ratio of right to left ventricular diameter of ≥0.9 on a computed tomography (CT) scan in a smaller sample of 1,002 randomly selected subjects; right ventricular dysfunction was identified in 35% of these subjects.8 The risk of recurrent VTE was reduced in the population with PE and elevated NT-proBNP (HR 0.52, 95% CI 0.28 to 0.98). Data from the smaller subset of patients with right ventricular dysfunction identified by CT scanning supported this finding, as the HR for VTE was 0.42 (95% CI 0.15 to 1.20).
A post-hoc analysis of the trial evaluated the relationship between right ventricular dysfunction and effects on recurrent VTE in more detail.9 More measurements at baseline were available for this analysis: 175 more patients had NT-proBNP measurements (95% of the study population underwent this measurement) and 881 more patients had measurement of right:left ventricular diameter (57% of all patients), each compared with the main trial analysis.8 There was an apparent reduction in the risk of recurrent VTE in favour of edoxaban in patients with PE and elevated NT-proBNP, but not in patients with PE but no elevation of NT-proBNP. There was also no apparent difference in the risk of recurrent VTE in the smaller populations of patients with or without recorded elevated right:left ventricular diameter, or in patients with or without both elevated NT-ProBNP and recorded elevated right:left ventricular diameter. The authors concluded that edoxaban may be more effective than warfarin in patients with PE and stable right ventricular function, and that edoxaban is suitable for use for prevention of recurrent PE irrespective of right ventricular function.
Other important post-hoc analyses from Hokusai-VTE
Descriptive analysis of major bleeding events
The incidence of major bleeding, a component of the main safety outcome was not reduced significantly by edoxaban versus warfarin/enoxaparin in the study, although there was a significant reduction in the risk of CRNM bleeds.8 An adjudicated analysis, conducted blinded to treatment, evaluated the nature of major bleeding events in the trial in terms of their clinical presentation (four categories ranged from non-emergency to causing death on or very soon after admission) and clinical course (a further four categories encompassing symptomatic treatment only, standard interventions such as transfusions, elaborate interventions for life-threatening events, and palliative care for events where the patient clearly could not be saved).10 The severest categories for clinical presentation of bleeds were assigned to 46% of major bleeds on edoxaban and 58% of major bleeds on warfarin (p=0.19). Corresponding incidences for the two most severe categories of clinical course were 23% for edoxaban and 29% for warfarin (p=0.46).
Dose and duration of treatment
Dose reductions were mandated by the trial protocol for certain patient characteristics, as described above.8 Of 1,452 patients with these characteristics, 733 were randomised to edoxaban (and received the 30 mg dose) and 719 patients were randomised to warfarin.11 Patients who received the reduced dose of edoxaban fared as well in the study as those who did not: HRs (95% CI) for main outcomes between these groups were 0.96 (0.61 to 1.52) for recurrent VTE, 0.99 (0.74 to 1.31) for clinically relevant bleeding, and 1.19 (0.62 to 2.3) for CRNM bleeding. Patients in the warfarin group who met the criteria for a dose reduction (had they been randomised to edoxaban) also had a similar risk of recurrent VTE as those who did not (HR 1.22, 95% CI 0.82 to 1.82). By contrast, they demonstrated increased risk of major/CRNM bleeding (HR 1.38, 95% CI 1.09 to 1.74) and major bleeding (HR 2.51, 95% CI 1.50 to 4.20), compared with patients who did not meet the criteria. The comparison between treatments was essentially unaltered from the main analysis, with comparable risk of recurrent VTE (HR 0.93, 95% CI 0.44 to 1.98) and reduced risk of major or CRNM bleeding (HR 0.62, 95% CI 0.44 to 0.86, p=0.004), CRNM bleeding (HR 0.6, 95% CI 0.42 to 0.87, p=0.007) or any bleeding (HR 0.6, 95% CI 0.55 to 0.83, p<0.001) in favour of edoxaban 30 mg, in the on-treatment analysis.
The main trial analyses included data from patients eligible for the on-treatment modified intention-to-treat (mITT) or overall intention-to-treat (ITT) analyses, irrespective of the duration of their study treatment.8 A post-hoc analysis stratified the population according to duration of treatment broken down into three-monthly time windows.12 The risk of recurrent VTE did not differ significantly between treatments for any of these time windows, or for treatment from >3 months to 12 months (HR 0.97, 95% CI 0.7 to 1.4, in the ITT analysis). Effects on the main safety end point of clinically relevant bleeding also did not differ between treatments over this time period (HR 0.97, 95% CI 0.77 to 1.22). In contrast, there were fewer major bleeds on edoxaban versus warfarin during this extended period of treatment (HR 0.45, 95% CI 0.22 to 0.92).
Demographic and disease characteristics
A set of subgroup analyses published together evaluated the impact of age, comorbidity and polypharmacy on treatment outcomes in Hokusai-VTE (figure 2).13 The incidence of the primary efficacy outcome occurred in similar proportions of patients aged <65 years, 65 to <75 years and ≥75 years (3.0%, 4.1% and 2.5%, respectively, p for trend 0.1839), but increased with increasing age in the warfarin group (3.0%, 4.4%, and 5.0%, respectively, p for trend 0.0294). Consistent with these observations, there was a trend for lower HRs in favour of edoxaban as the age category increased (1.00, 0.97 and 0.52, respectively), although pint was not significant (pint=0.16). Rates for major bleeding or clinically relevant non-major bleeding increased with age category in both groups (7.5%, 9.0%, and 12.5%, respectively, for edoxaban, p for trend 0.0005; and 8.6%, 12.5%, and 15.1% respectively, for warfarin, p for trend <0.0001), again with no significant interaction between age category and outcomes (pint=0.50). Similar results for efficacy and safety outcomes were obtained when age 80 years was used as the cut-off value.
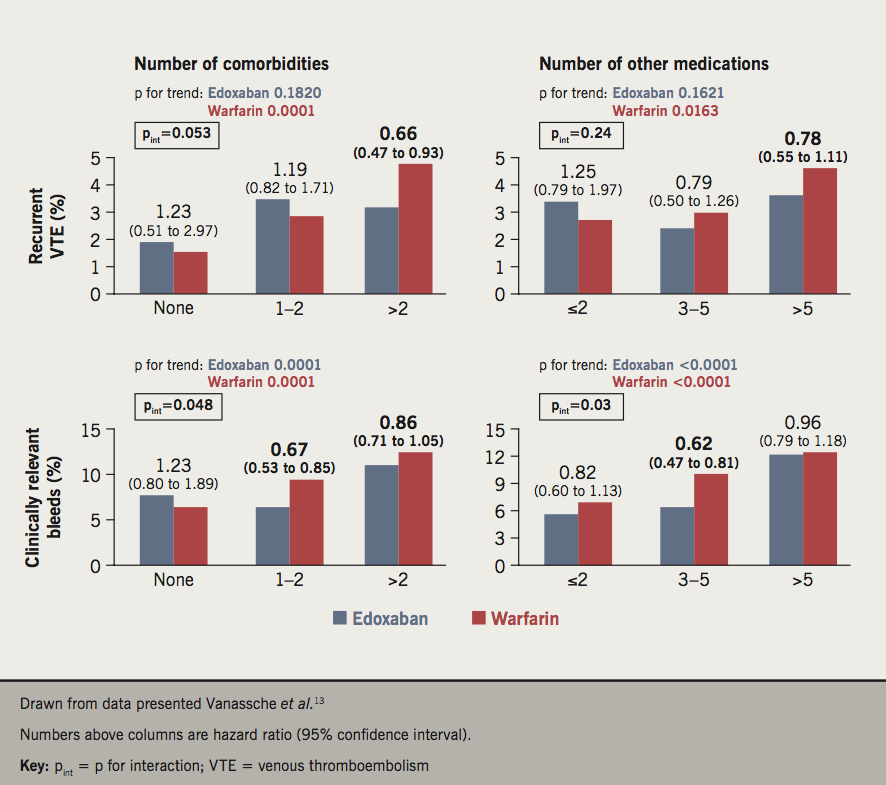
Similar results were obtained when patients were stratified according to increasing number of comorbidities (none, one or two, and more than two) and number of concomitant medications (two or less, three to five, and more than five). In each case, there was a significant trend for increasing incidence of recurrent VTE on warfarin but not edoxaban, and a significant trend for more major bleeding for both treatments, as these categories increased, without any significant treatment interactions. A net benefit outcome, incidence of VTE or major bleeding, increased in incidence with increasing values of age, comorbidity and polypharmacy similarly for both treatments.
The Hokusai-VTE trial population included 1,109 patients from Japan, China, Korea, and Taiwan.8,14 Efficacy and safety findings in this East Asian population were similar to those in the overall analysis: the HRs (95% CI) for the main efficacy outcome of recurrent VTE and the main safety outcome of clinically relevant bleeding were 0.64 (0.34 to 1.19), p=0.1601, and 0.56 (0.40 to 0.78), p< 0.001, respectively.14
Management of VTE in the outpatient setting
Slightly more than one-quarter (27.1%) of the Hokusai-VTE population with known hospital admission status and length of stay were managed as outpatients.15 Compared with those hospitalised for more than five days, patients managed at home were more likely to have DVT (83.0% vs. 48.8%), and were less likely to have PE (17% vs. 51.2%). Unsurprisingly, patients managed at home contained higher proportions with limited or intermediate severity, and lower proportions with extensive severity, for their qualifying DVT and PE. Patients managed at home were also younger, and with better renal function, on average, and fewer managed at home than in hospital qualified for the edoxaban dose reduction. There were no significant differences between study treatments in patients managed at home, for either the rate of recurrent DVT (risk difference 0.28, 95% CI –2.68 to 3.24) or clinically relevant bleeding (risk difference 0.36, 95% CI –1.45 to 0.73).
Discussion and clinical implications of the Hokusai-VTE study
The analysis of the nature of major bleeds on edoxaban demonstrated that these events were similar in presentation and clinical course to those occurring on a VKA, which should increase confidence in prescribing this agent.10 The main risk factors for bleeding events in Hokusai-VTE were female gender, concomitant antiplatelet therapy, anaemia (haemoglobin ≤10 g/dL), an existing diagnosis of hypertension, and current elevated systolic blood pressure (>160 mmHg).16 The observations of preserved efficacy and reduced major bleeding during extended treatment for edoxaban versus warfarin,12 and a preserved therapeutic profile in patients who require a dose reduction of edoxaban,11 are also reassuring. The observation that patients with milder presentation of initial VTE can be successfully managed at home has the potential to facilitate home-based treatment of these patients, in line with current management guidelines, facilitated by the more straightforward administration of a NOAC, compared with a VKA.15 Finally, increasing age, comorbidity and polypharmacy appeared to identify a vulnerable population at increased risk of clinically relevant bleeding (both treatment groups) and recurrent VTE (observed only in the warfarin group).13 Other studies have previously identified these patient characteristics as risk factors for VTE.17,18 Importantly, edoxaban was as effective, with a similar safety profile, as warfarin in all of these subgroups.13
The right ventricle is designed to operate against a low arterial resistance, but a substantial PE may increase right ventricular afterload, resulting in right ventricular dysfunction. It has been estimated that half or more of patients admitted with PE have acute or chronic right ventricular dysfunction, characterised by right ventricular pressure overload.19,20 We have known for decades that occlusion of even less than 25% of the pulmonary bed can cause clinically significant increases in pulmonary arterial pressure.21 Right ventricular dysfunction is strongly prognostic for adverse outcomes in PE, and a recent study from the USA found a nine-fold increase in early mortality in patients with or without right ventricular dysfunction.20 Cardiac biomarkers, such as troponin and NT-proBNP, are elevated in this setting and also predict mortality.22,23 Clinical data are limited on the effects of NOACs in populations with patients with PE and right ventricular dysfunction.24 The data from Hokusai-VTE are welcome in this regard, but more studies in this area are needed.
The successful management of low-risk patients with VTE at home (as evidenced by comparable event rates with those managed in hospital) was an important finding from the study, and is consistent with guideline-based care. The management of PE is based on assessment of the risk of mortality, according to European guidance.25 High-risk patients present with shock or hypotension. Intermediate risk patients have a Class III–V pulmonary embolism severity index (PESI) score (or simplified PESI [sPESI] score ≥I), with evidence of right heart dysfunction and/or elevated cardiac biomarkers. Anticoagulation in hospital is recommended for patients at intermediate risk. Patients identified as being at low mortality risk (PESI Class I or II score) may be discharged early and treated with an anticoagulant at home. Current European clinical guidance recommends treatment at home for the majority of patients with DVT.26 However, recent evidence from Italy showed that about half of patients with DVT and about five-sixths of patients with PE were managed in hospital, and that a low-risk PESI score was not a significant predictor of where patients were managed.27
Conclusion
Edoxaban was as effective as warfarin for preventing recurrent VTE in patients presenting with DVT or PE, and was associated with fewer major bleeding events than warfarin, in the randomised Hokusai-VTE study. Moreover, edoxaban was as effective as warfarin, with a similar safety profile, in a range of subgroups of patients.
Key messages
- The randomised Hokusai-VTE study compared the non-vitamin K antagonist oral anticoagulant, edoxaban, with warfarin in 8,240 patients with venous thromboembolism (VTE)
- Edoxaban was non-inferior to warfarin for preventing recurrent VTE (the primary end point of the study) and was associated with significantly less major bleeding, and any bleeding, than warfarin
- The therapeutic profile of edoxaban was maintained in important patient subgroups, including those based on demographic an disease characteristics, including the presence of right heart dysfunction
Conflicts of interest
JT has received honoraria from Daichii Sankyo, Bayer, BMS-Pfizer and Boehringer Ingelheim.
Acknowledgement
A medical writer (Dr Mike Gwilt, GT Communications) provided editorial assistance, funded by the British Journal of Cardiology.
Jecko Thachil
Consultant Haematologist
Manchester Royal Infirmary, Manchester University NHS Foundation Trust, Oxford Road, Manchester, M13 9WL
Email: jecko.thachil@mft.nhs.uk
Articles in this supplement
Introduction
1. Insights in patients with atrial fibrillation and co-existing cardiovascular disease
2. Insights in stroke prevention in patients with a prior history of stroke or transient ischaemic attack
4. Insights from primary care: opportunities and challenges
References
1. National Institute for Health and Care Excellence. Clinical knowledge summary. Pulmonary embolism. London: NICE, 2015. Available from: https://cks.nice.org.uk/pulmonary-embolism
2. National Institute for Health and Care Excellence. Clinical knowledge summary. Deep vein thrombosis. London: NICE, 2018. Available from: https://cks.nice.org.uk/deep-vein-thrombosis
3. Thrombosis UK. Thrombosis statistics. Available at: https://www.thrombosisuk.org/thrombosis-statistics.php [accessed March 2019].
4. National Health Service England. Venous thromboembolism (VTE) risk assessment. Available at: https://www.england.nhs.uk/statistics/statistical-work-areas/vte [accessed March 2019].
5. Patel R. Effective management of venous thromboembolism in the community: non-vitamin K antagonist oral anticoagulants. Int J Gen Med 2016;9:107–15. https://doi.org/10.2147/IJGM.S100299
6. National Institute for Health and Care Excellence. BNF content published by NICE. Interactions. Warfarin. Available at: https://bnf.nice.org.uk/interaction/warfarin.html [accessed March 2019].
7. Sindet-Pedersen C, Pallisgaard JL, Staerk L et al. Temporal trends in initiation of VKA, rivaroxaban, apixaban and dabigatran for the treatment of venous thromboembolism – a Danish nationwide cohort study. Sci Rep 2017;7:3347. https://doi.org/10.1038/s41598-017-03596-x
8. Hokusai-VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15. https://doi.org/10.1056/NEJMoa1306638
9. Brekelmans MP, Ageno W, Beenen LF et al. Recurrent venous thromboembolism in patients with pulmonary embolism and right ventricular dysfunction: a post-hoc analysis of the Hokusai-VTE study. Lancet Haematol 2016;3:e437–e445. https://doi.org/10.1016/S2352-3026(16)30080-1
10. Brekelmans MP, Bleker SM, Bauersachs R et al. Clinical impact and course of major bleeding with edoxaban versus vitamin K antagonists. Thromb Haemost 2016;116:155-61. https://doi.org/10.1160/TH15-11-0892
11. Verhamme P, Wells PS, Segers A et al. Dose reduction of edoxaban preserves efficacy and safety for the treatment of venous thromboembolism. An analysis of the randomised, double-blind Hokusai VTE trial. Thromb Haemost 2016;116:747–53. https://doi.org/10.1160/TH16-03-0244
12. Raskob G, Ageno W, Cohen AT et al. Extended duration of anticoagulation with edoxaban in patients with venous thromboembolism: a post-hoc analysis of the Hokusai-VTE study. Lancet Haematol 2016;3:e228–e236. https://doi.org/10.1016/S2352-3026(16)00023-5
13. Vanassche T, Verhamme P, Wells P et al. Impact of age, comorbidity, and polypharmacy on the efficacy and safety of edoxaban for the treatment of venous thromboembolism: an analysis of the randomized, double-blind Hokusai-VTE trial. Thromb Res 2018;162:7–14. https://doi.org/10.1016/j.thromres.2017.12.005
14. Nakamura M, Wang YQ, Wang C et al. Efficacy and safety of edoxaban for treatment of venous thromboembolism: a subanalysis of East Asian patients in the Hokusai-VTE trial. J Thromb Haemost 2015;13:1606–14. https://doi.org/10.1111/jth.13055
15. Medina A, Raskob G, Ageno W et al. Outpatient management in patients with venous thromboembolism with edoxaban: a post hoc analysis of the Hokusai-VTE study. Thromb Haemost 2017;117:2406–14. https://doi.org/10.1160/TH17-05-0523
16. Di Nisio M, Raskob G, Büller HR et al. Prediction of major and clinically relevant bleeding in patients with VTE treated with edoxaban or vitamin K antagonists. Thromb Haemost 2017;117:784–93. https://doi.org/10.1160/TH16-11-0830
17. Premuš Marušič A, Petrovič D, Mrhar A, Locatelli I. Polypharmacotherapy and blood products as risk factors for venous thromboembolism in postsurgical patients: a case-control study. Int J Clin Pharm 2017;39:416–23. https://doi.org/10.1007/s11096-017-0441-7
18. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol 2015;12:464–74. https://doi.org/10.1038/nrcardio.2015.83
19. Bryce YC, Perez-Johnston R, Bryce EB, Homayoon B, Santos-Martin EG. Pathophysiology of right ventricular failure in acute pulmonary embolism and chronic thromboembolic pulmonary hypertension: a pictorial essay for the interventional radiologist. Insights Imaging 2019;10:18. https://doi.org/10.1186/s13244-019-0695-9
20. Witkin A, Wilcox SR, Chang Y et al. Impact of chronic right ventricular pressure overload in short-term outcomes of acute pulmonary embolism: a retrospective analysis. J Crit Care 2019;51:1–5. https://doi.org/10.1016/j.jcrc.2019.01.007
21. Alpert JS, Godtfredsen J, Ockene IS, Anas J, Dalen JE. Pulmonary hypertension secondary to minor pulmonary embolism. Chest 1978;73:795–7. https://doi.org/10.1378/chest.73.6.795
22. Barco S, Mahmoudpour SH, Planquette B, Sanchez O, Konstantinides SV, Meyer G. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J 2019;40:902–10. https://doi.org/10.1093/eurheartj/ehy873
23. Jia D, Liu F, Zhang Q, Zeng GQ, Li XL, Hou G. Rapid on-site evaluation of routine biochemical parameters to predict right ventricular dysfunction in and the prognosis of patients with acute pulmonary embolism upon admission to the emergency room. J Clin Lab Anal 2018;32:e22362. https://doi.org/10.1002/jcla.22362
24. Gómez-Outes A, Suárez-Gea ML, Lecumberri R, Terleira-Fernández AI, Vargas-Castrillón E. Direct oral anticoagulants in the treatment of venous thromboembolism, with a focus on patients with pulmonary embolism: an evidence-based review. Vasc Health Risk Manag 2014;10:627–39. https://doi.org/10.2147/VHRM.S50543
25. Konstantinides SV, Torbicki A, Agnelli G et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033–69, 3069a–3069k. https://doi.org/10.1093/eurheartj/ehu283
26. Mazzolai L, Aboyans V, Ageno W et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur Heart J 2018;39:4208–18. https://doi.org/10.1093/eurheartj/ehx003
27. Dentali F, Di Micco G, Giorgi Pierfranceschi M et al. Rate and duration of hospitalization for deep vein thrombosis and pulmonary embolism in real-world clinical practice. Ann Med 2015;47:546–54. https://doi.org/10.3109/07853890.2015.1085127


