Stent edge dissection is one of the procedural complications concerning percutaneous coronary intervention (PCI). We present a clinical case of multi-vessel PCI where the patient had to return with recurring symptoms within two weeks of a seemingly successful PCI, only to teach us a valuable lesson in the more frequent and judicious use of intracoronary imaging.
Introduction
Coronary angiography and intravascular ultrasound (IVUS) have traditionally been used for diagnosis of stent edge dissection.1 Optical coherence tomography (OCT), with better resolution (12 to 18 µm) as compared with IVUS (150–250 µm), has higher chances of detecting stent edge dissection. Reported incidences of stent edge dissection range from 5% to 23% by IVUS,2 compared with 20% to 56% by OCT.3 Angiographic results are often deceiving and trials have confirmed that OCT changes the initial PCI strategy in a significant number of cases.4
Case summary
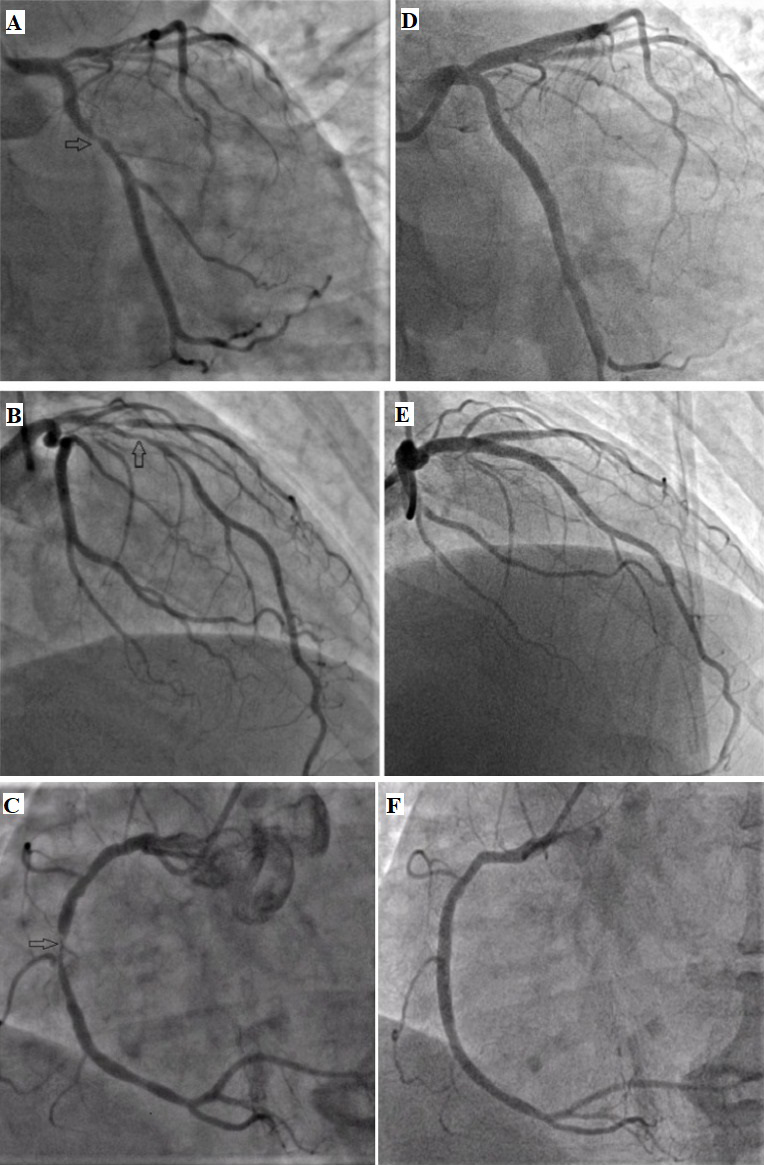
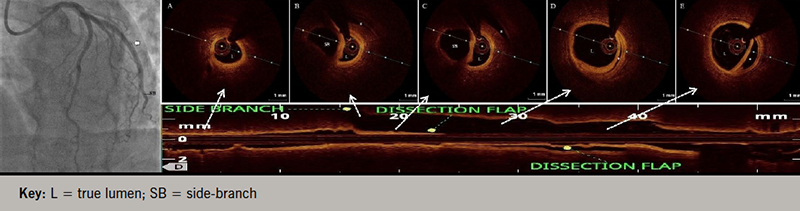
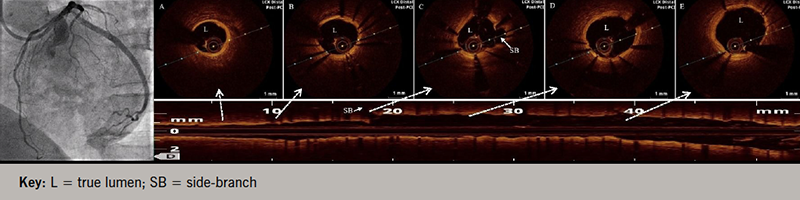
A 58-year-old, non-diabetic, non-hypertensive, tobacco-chewing man presented with the complaint of angina on exertion – Canadian Cardiovascular Society (CCS) class II for the last two years with recent worsening to class III – refractory to maximally tolerated anti-anginal therapy. His physical examination was unremarkable, with normal resting electrocardiogram (ECG) and echocardiogram. Coronary angiogram revealed triple-vessel disease for which he underwent percutaneous coronary intervention (PCI) to left circumflex artery (LCx) with a 3.5 × 20 mm sirolimus-eluting stent (video 1), dedicated left anterior descending (LAD) artery diagonal bifurcation and right coronary artery (RCA) with good angiographic results (figure 1, videos 2 and 3). Post-stenting, TIMI (Thrombolysis in Myocardial Infarction) 3 flow was achieved with no procedure-related complication. However, two weeks post-procedure, the patient complained of progressively increasing effort angina, CCS class III. No new ST-T segment changes or regional wall motion abnormality was detected. Serum troponin levels were also within normal range. Angiogram revealed patent stents except for a long-segment coronary artery stent edge dissection in LCx extending into the distal vessel with TIMI 2 flow (video 4). Optical coherence tomography (OCT) pullback revealed a spiral dissection extending into media involving a more than 180 degrees arc of vessel circumference without any evidence of thrombus, and sparing side branches with significant luminal compromise (minimum luminal area of 1.50 mm2) (figure 2, video 5). Coronary diameter of proximal LCx was estimated to be 3.0 mm, following which an everolimus-eluting stent (2.75 × 48 mm) was implanted under OCT guidance with good angiographic results, achieving a proximal mean luminal area of 5.58 mm2 (figure 3, videos 6 and 7). At two-year follow-up, the patient is asymptomatic with no functional limitation.
Discussion
This case demonstrates the importance of OCT in delineating stent failure, edge dissection and optimal landing zones that were unfortunately missed in the index procedure, which in due course of time led to the luminal compromise and worsening of angina. An OCT-guided PCI strategy has been shown to improve cardiovascular (CV) outcomes, specifically CV death and myocardial infarction, by playing a significant role in optimising PCI results by identifying stent under-expansion, mal-apposition, plaque protrusion, and edge dissections.4 Stent edge dissections are characterised by unplanned vessel tearing that occurs at the transition between the rigid stent cage and the adjacent arterial wall – a region of compliance mismatch. Edge dissections are defined as disruptions of the arterial lumen surface in the stent edge segments (either 5 mm proximal or distal to the stent borders). Key predictors for stent edge dissection are stent borders positioned over diseased reference segments (particularly with eccentric plaques), certain plaque types (e.g. calcified, lipid-rich, and thin cap fibroatheroma [TCFA]), morphometry at the stent landing zone (e.g. calcium angle and fibrous cap thickness), stent and lumen eccentricity, and mismatch between stent and lumen dimensions.5 The vast majority (84%) of the OCT-detected edge dissections are not apparent on angiography.6 Based on OCT findings, criteria laid down for advocating intervention in coronary dissection are: ≥200 µm wide edge dissection, extending beyond intima, dissection flap involving circumferential arc of ≥60º, flow-limiting dissection or dissection noted in more than three frames. In our case, dissection flap was missed angiographically in the index procedure, but during the second procedure OCT clearly illuminated the underlying patho-anatomic abnormality, thereby leading to its optimal and timely management. The 2018 European Society of Cardiology guidelines7 for myocardial revascularisation, therefore, advocates considering the use of OCT in selected patients for optimising stent implantation (class of recommendation IIa, level of evidence B).
Conclusion
Stent edge dissections are procedural complications and are usually benign. In our patient, an angiographically silent stent edge dissection progressed within two weeks leading to flow compromise. OCT helped to define the precise extent and clinical significance of stent edge dissection, proximal and distal reference vessel diameter and prevent iatrogenic stent overexpansion. OCT clearly edges over conventional angiography in identifying stenting-related complications, understanding their patho-anatomy and resolving these issues. Therefore, intravascular imaging, preferably OCT, should be employed frequently to identify the underlying mechanisms and guide probable treatment options for post-stenting coronary artery dissections.
Conflicts of interest
None declared.
Funding
None.
Patient consent
Appropriate written informed consent for interventional procedure and subsequent publication of patient’s clinical and angiographic imaging details was obtained.
Editors’ note
Videos available from author on request:
Video 1. Diagnostic angiogram (AP caudal view) showing lesion in left circumflex artery
Video 2. Angiographic result of left circumflex artery post-PCI (AP caudal view)
Video 3. Angiographic result of left circumflex artery post-PCI (LAO view)
Video 4. Check angiogram (LAO view) showing dissection in left circumflex artery
Video 5. OCT pullback confirming coronary dissection in left circumflex artery
Video 6. Final angiogram post-stenting (LAO view)
Video 7. Post-stenting OCT pullback confirming optimal coverage of dissection by stent strut without any mal-apposition or residual dissection
References
1. Holmes DR, Holubkov R, Vlietstra RE et al. Comparison of complications during percutaneous transluminal coronary angioplasty from 1977 to 1981 and from 1985 to 1986: the National Heart, Lung, and Blood Institute percutaneous transluminal coronary angioplasty registry. J Am Coll Cardiol 1988;12:1149–55. https://doi.org/10.1016/0735-1097(88)92593-4
2. Sheris SJ, Canos MR, Weissman NJ. Natural history of intravascular ultrasound-detected edge dissections from coronary stent deployment. Am Heart J 2000;139:59–63. https://doi.org/10.1016/S0002-8703(00)90309-0
3. Radu MD, Räber L, Heoet J et al. Natural history of optical coherence tomography-detected nonflow-limiting edge dissections following drug-eluting stent implantation. EuroIntervention 2014;9:1085–94. https://doi.org/10.4244/EIJV9I9A183
4. Prati F, Vito LD, Biondi-Zoccai G et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l’Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention 2012;8:823–9. https://doi.org/10.4244/EIJV8I7A125
5. Gonzalo N, Serruys PW, Okamura T et al. Optical coherence tomography assessment of the acute effects of stent implantation on the vessel wall: a systematic quantitative approach. Heart 2009;95:1913–19. https://doi.org/10.1136/hrt.2009.172072
6. Chamie D, Bezerra HG, Attizzani GF et al. Incidence, predictors, morphological characteristics, and clinical outcomes of stent edge dissections detected by optical coherence tomography. JACC Cardiol Interv 2013;6:800–13. https://doi.org/10.1016/j.jcin.2013.03.019
7. Neumann FJ, Sousa-Uva M, Ahlsson A et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. https://doi.org/10.1093/eurheartj/ehy394
