In order to evaluate the extent and causes of pain during cardiac implantable electronic device (CIED) implantation in our hospital, a prospective audit over a 23-month period using a patient self-reporting questionnaire was undertaken.
In total, 599 procedures were reported, 52.9% for de novo pacemaker implantation and 23.4% for high-energy devices (cardiac resynchronisation therapy defibrillator [CRT-D], implantable cardiac defibrillator [ICD], subcutaneous ICD). Overall, the median pain score was 2/10 (interquartile range 2–4). In total, 61.6% (367/599) reported no pain or mild pain (pain scores 0–3/10), 27.7% (165/599) reported moderate pain (pain score of 4–6/10) and 10.7% (64/599) reported severe pain (pain score of 7–10/10) during the procedure. Significant pre-implant worry (odds ratio [OR] 2.13, 95% confidence interval [CI] 1.22 to 3.73) and higher lidocaine doses (OR 1.06, 95%CI 1.00 to 1.11) were associated with severe patient-reported pain.
In conclusion, most patients underwent CIED implantation with minimum stress and maximum comfort. An important minority reported severe pain during the procedure. Optimising surgical technique and interventions targeted at reducing pre- and peri-implant worry, particularly in women, and especially in those receiving ICDs, warrants further investigation to reduce patient-reported pain during CIED implantation.
Introduction
Cardiac implant electronic devices (CIED) are undertaken under local anaesthetic by cardiologists. Patient experience is a key metric by which the delivery of healthcare is assessed, and, for CIED implantation, optimal peri-procedural pain management is central to patient experience. A recent study has highlighted that significant procedural pain in CIED implantation is underestimated and poorly predicted.1 According to the James Lind Alliance, a collaboration of British patients, carers and clinicians that set research priorities in anaesthesia and peri-operative medicine, several research themes relating to improving patient experience, reducing anxiety in the lead up to an operation and to improving peri-operative pain and recovery have been deemed to be important.2 These research priorities are applicable to CIED implantation but are seldom prioritised. Most research in the field of cardiac rhythm management in the past has centred on technology and its application, rather than on patient experience, and future research is also likely to focus on how to maximise the benefit of technology within a patient population, rather than on patient experience per se.3,4
This study is designed to evaluate the extent and causes of pain during CIED implantation in our hospital.
Method
A prospective audit was carried out of CIED implants performed at a single centre between December 2016 and December 2019 using a patient self-report questionnaire. Following the CIED implant, a cardiac physiologist administered the questionnaire, which asked patients to score their level of worry prior to the procedure, their pain during the procedure on a 0–10 visual analogue scale and whether the pain matched their expectations. A low score represents a low level of worry or pain with 0/10 representing no worry or pain. A high score represents a high level of worry or pain with 10/10 representing maximal worry or pain. This visual analogue scale has not been validated in a CIED population but has been extensively studied and shown to be an acceptable, valid and reliable measure of pain intensity in other fields.5
Procedures included permanent pacemaker (PPM), cardiac resynchronisation therapy pacemaker (CRT-P), cardiac resynchronisation therapy defibrillator (CRT-D), implantable cardiac defibrillator (ICD), lead revisions and generator changes (including upgrading and downgrading of devices). Data were collected on patient demographics and intra-operative use of sedation and local anaesthesia.
Procedures were undertaken by five operators; four consultant cardiologists and one competent non-consultant cardiologist. In cases where a trainee cardiologist assisted a cardiologist, the cardiologist rather than the trainee was recorded as the operator. All cardiologists had performed at least 300 CIED implants. All operators were aware of the ongoing audit. All procedures were performed in Worcestershire Royal Hospital.
Use of local anaesthesia, intra-operative intravenous anxiolytic and intra-operative intravenous opioid analgesia was at the discretion of the operator. Pre-operative sedation (pre-med) is not routinely used in this hospital.
Statistical analysis
Table 1. Baseline characteristics
| Number (N=599) |
Percentage (IQR) |
|
|---|---|---|
| Median age, years | 77 | (71.5 – 83.8) |
| Male gender | 411 | 68.7 |
| Pacemaker | 317 | 52.9 |
| ICD | 56 | 9.4 |
| CRT-P | 64 | 10.7 |
| CRT-D | 72 | 12.0 |
| Upgrade to CRT-D | 18 | 3.0 |
| Various* | 17 | 2.8 |
| Generator exchange | 43 | 7.2 |
| S-ICD | 12 | 2.0 |
| High-energy device** | 140 | 23.4 |
| Operator | ||
| One | 101 | 16.86 |
| Two | 304 | 50.75 |
| Three | 105 | 17.53 |
| Four | 55 | 9.18 |
| Five | 34 | 5.68 |
| * Various includes new pace/sense lead, wound revision and lead revision, downgrade to CRT-P, lead removal and ILR removal **High-energy device comprises ICD, CRT-D and S-ICD Key: CRT-D = cardiac resynchronisation therapy and defibrillator; CRT-P = cardiac resynchronisation therapy and pacemaker; ICD = implantable cardioverter defibrillator; ILR = implantable loop recorder; IQR = interquartile range; S-ICD = subcutaneous implantable cardioverter defibrillator |
||
Continuous variables are expressed as the mean ± standard deviation (SD) and were compared using Student’s t-test or nonparametric analysis where appropriate. A two-tailed p value of <0.05 indicated statistical significance. The continuous variables ‘worry scale 0–10’ and ‘pain 0–10’ were dichotomised into severe for scores of seven and over and non-severe for scores less than seven. A univariate regression analysis was undertaken to evaluate the predictors of severe pain. Variables were selected with the backward stepwise method using a cut-off probability value for inclusion and exclusion of 0.10. A multi-variate logistic regression analysis was then performed to assess the influence of these variables as independent risk factors for severe pain during CIED implantation. The odds ratio (OR), corresponding 95% confidence interval (CI), and the p value are reported for each independent factor. A logistic regression to identify predictors of severe pre-implant worry using the steps described above was then created using the coefficients of the regression analysis to estimate individual patient’s risk. Statistical analysis was performed using the STATA statistical package (Stata/IC 13.0 for Windows, StataCorp LP, USA).
Results
Baseline data are summarised in table 1. In total, 599 procedures were reported of which 52.9% were for de novo pacemaker implantation and 23.4% were for high-energy devices (ICD, CRT-D and subcutaneous ICD [S-ICD]).
In all cases, lidocaine 1% was the local anaesthetic that was used, unless the device was a S-ICD or if a subpectoral pocket was fashioned, where prilocaine 1% was used. Intravenous anxiolytics were used in 73.1% of all cases (table 2). Operators used similar rates of local anaesthesia (range from mean 18.6 ml for operator 3 to 21.7 ml for operator 1) but there was more variation in the use of intravenous anxiolytic (range from 48% for operator 3 to 86% of cases for operator 2) and intravenous opioid analgesia (range from 2% for operator 4 to 30% of cases for operator 2).
Table 2. Use and median doses of local anaesthesia, intra-operative intravenous anxiolytic and intra-operative intravenous opioid analgesia
| Number | Percentage | Median dose | IQR | |
|---|---|---|---|---|
| 1% lidocaine, ml* | 568 | 94.8 | 20 | 19 – 22 |
| 1% prilocaine, ml* | 12 | 5.2 | 60 | 50 – 100 |
| Sedation use | 438 | 73.1 | ||
| Midazolam, mg | 352 | 58.7 | 1 | 0 – 2 |
| Diazemuls, mg | 62 | 10.3 | 2.5 | 2.5 – 5 |
| Morphine sulphate, mg | 56 | 9.3 | 5 | 5 – 7.15 |
| Fentanyl, µg | 82 | 13.7 | 50 | 25 – 50 |
| *In total, 568 patients received just lidocaine, 12 patients received just prilocaine, 1 patient received both lidocaine and prilocaine and this patient not included in dose calculation. There were 26 instances where the local anaesthetic that was used was not recorded. It is likely that all of these cases used lidocaine as none of the cases involved a subcutaneous implantable cardioverter defibrillator (S-ICD). Key: IQR = interquartile range |
||||
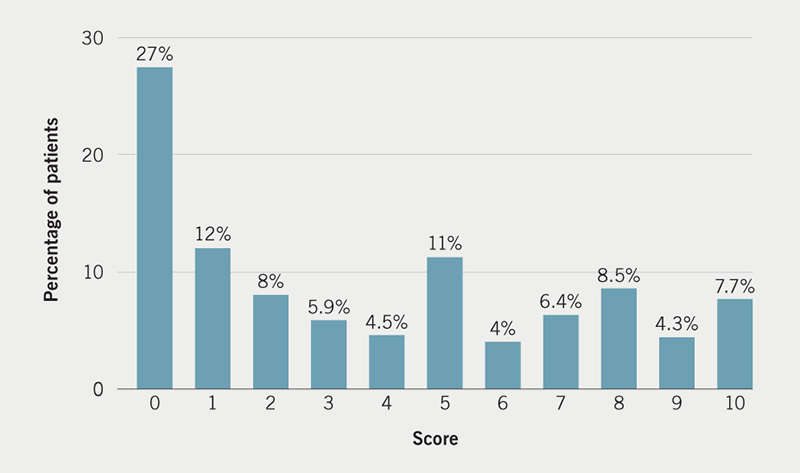
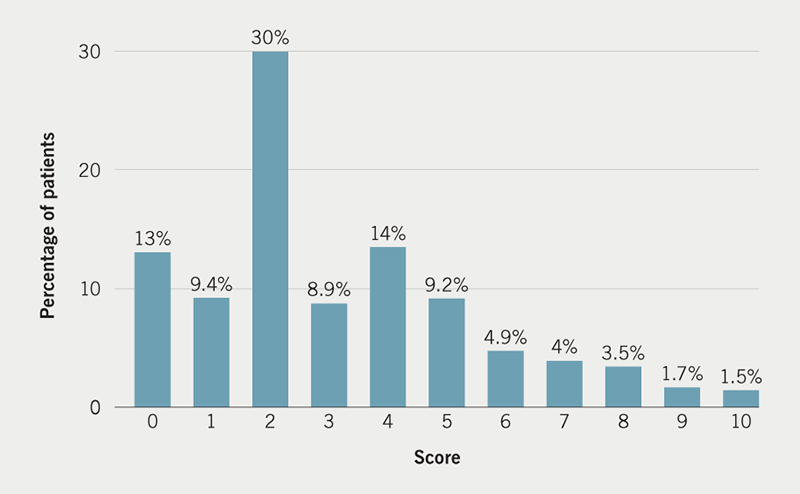
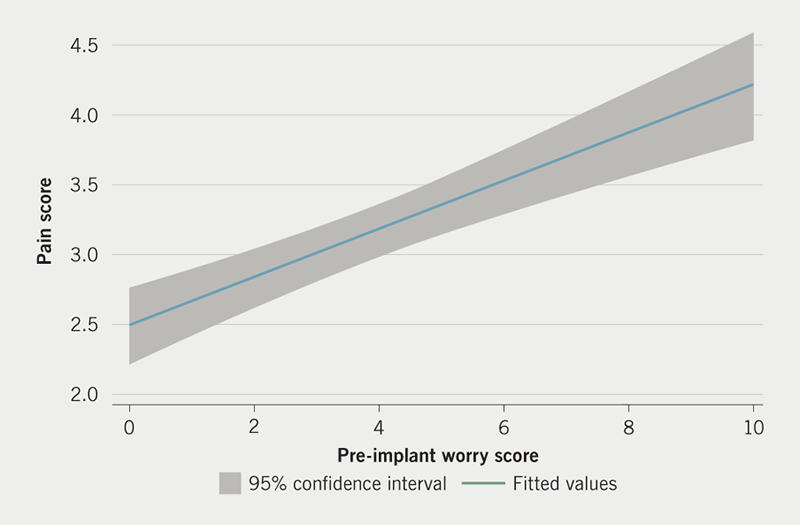
Patient-reported pre-implant worry is shown in figure 1. The median score was 3/10 although 161/599 (26.9%) reported severe levels of worry with a score of 7/10 or higher. Overall, the median pain score was 2/10 (interquartile range 2–4). In total, 61.6% (367/599) reported no pain or mild pain (pain scores 0–3/10), 27.7% (165/599) reported moderate pain (pain score of 4–6/10) and 10.7% (64/599) reported severe pain (pain score of 7–10/10) during the procedure (figure 2). The relationship between patient pre-implant worry and patient-reported pain during CIED implantation is presented in figure 3. In total, 47.9% (287/599) of patients reported intra-operative pain to be less than expected, 29.2% (175/599) of patients reported the pain to be as they expected and 22.8% (137/599) reported the pain to be more than they had expected.
Predictors of pain
A univariate logistic regression analysis of predictors of patient-reported severe pain that were included in the multi-variate model is presented in table 3. The results of the multi-variate analysis are presented in table 4. Male gender and operator 2 were identified as predictors of lower patient-reported pain, and significant levels of pre-implant worry and higher lidocaine doses were identified as predictors of severe patient-reported pain. The logistic regression model was statistically significant (χ2=27.4, p<0.001), explaining 6.4% (pseudo R2) of the variance in severe pain.
Table 3. Univariate predictors of patient-reported severe pain
| Odds ratio | p value | 95% confidence interval | |
|---|---|---|---|
| Male gender | 0.42 | 0.001 | 0.25 to 0.72 |
| Severe pre-implant worry | 2.37 | 0.001 | 1.40 to 4.04 |
| Operator 1 | 1.93 | 0.032 | 1.06 to 3.52 |
| Operator 2 | 0.47 | 0.007 | 0.27 to 0.81 |
| Lidocaine dose | 1.05 | 0.041 | 1.00 to 1.11 |
Table 4. Multi-variate predictors of patient-reported severe pain
| Odds ratio | p value | 95% confidence interval | |
|---|---|---|---|
| Male gender | 0.47 | 0.007 | 0.27 to 0.81 |
| Severe pre-implant worry | 2.13 | 0.008 | 1.22 to 3.73 |
| Operator 2 | 0.54 | 0.03 | 0.30 to 0.94 |
| Lidocaine dose | 1.06 | 0.034 | 1.00 to 1.11 |
| Constant | 0.06 | 0 | 0.02 to 0.19 |
Predictors of pre-implant worry
A univariate logistic regression analysis of predictors of high levels of pre-implant worry (7/10 and higher) that were included in the multi-variate model is presented in table 5. Older age and male gender are associated with a lower likelihood of significant self-reported worry, and the need for a complex device is associated with higher likelihood of significant self-reported worry.
Table 5. Univariate predictors of patient-reported severe pre-implant worry
| Odds ratio | p value | 95% confidence interval | |
|---|---|---|---|
| Age | 0.96 | 0.000 | 0.95 to 0.98 |
| Male gender | 0.51 | 0.001 | 0.35 to 0.75 |
| Permanent pacemaker | 0.60 | 0.006 | 0.41 to 0.86 |
| Complex device | 1.93 | 0.000 | 1.33 to 2.78 |
Subcutaneous ICD implantation
The pain scores for the S-ICD cohort are consistent with the rest of the study population. Twelve patients received an S-ICD, all undertaken by operator 2, and all patients had defibrillation safety margin testing. Serratus nerve block was not used in this S-ICD cohort. Pain score was missing in one patient (overall, 3/599 pain scores were missing). In total, 1/11 experienced severe pain, median pain score 2 (IQR 2–4) versus 2 (IQR 2–5) for the whole population. A greater proportion of patients reported their pain to be less than expected (75% vs. 48% of the whole cohort), although a similar proportion reported their pain to be more than expected (25% vs. 23%). The S-ICD cohort, however, reported significantly higher rates of worry compared with the rest of the population (mean 6.66 vs. 3.66).
The S-ICD group also received higher doses of prilocaine (73 ml vs. approximately 20 ml 1% lidocaine for non-S-ICD interventions), higher doses of intravenous anxiolytics (median dose 8 mg vs. 1 mg) and higher doses of intravenous opioids (median dose 7.5 mg morphine vs. 5 mg).
Discussion
This study has evaluated the extent of patient-reported pain during CIED implantation in our hospital and explored potential causes of this, in particular with regard to patient-reported levels of worry prior to the procedure. This is the first report in the literature evaluating the link between patient-reported worry and procedural pain during CIED implantation. Overall, patient-reported pain during CIED implantation was low (median 2/10). However, a significant minority of patients, 10.7%, reported experiencing severe pain during the implant procedure. These findings were also seen in a small subgroup of patients who had S-ICD implanted under conscious sedation, although this group received much higher doses of local anaesthesia and sedation compared with the rest of the cohort.
Patient-reported pain scores in this study are consistent with those reported in another report of peri-operative pain management during cardiac electronic device implantation.6 In a recent study, the maximal pain during the implant was reported as 8/10 with a mean of approximately 3.3/10 but the pain gradually reduced over the following 24 hours. The authors did not report what proportion of patients experienced severe pain. Our study is important because we suspect that patient-reported pain during CIED implantation is more prevalent than previously suspected. Our results are consistent with an analysis of 16,500 adult patients who underwent CIED procedures in the US, of whom 20% were prescribed opioid analgesia.7 Of these, 80% were opioid naïve and 9% went on to have repeat opioid prescriptions. The authors of the study stated that peri-operative pain management in CIED procedures warrants attention, and that other studies on non-pharmacologic methods of pain relief, such as cognitive behavioural therapy would be important. The data presented in these two papers support the results we present here and, therefore, we believe that our results are generalisable to other centres that implant CIEDs.
In a logistic regression analysis, we have found that high levels of patient-reported pre-implant worry is associated with a two-fold increase in the odds of patient-reported severe pain during CIED implantation. While this is a novel observation in the field of CIED, this association is known in non-cardiac surgical specialities, such as orthopaedic surgery.8 In a systematic review of predictors of pain following hip or knee arthroplasty, authors reported a strong association between post-surgical pain and the following pre-surgical factors: female gender, low socio-economic status, higher pain, comorbidities, low back pain, poor functional status, and psychological factors (depression, anxiety or catastrophic pain). In our study, patients who were more worried were likely to be younger, female and undergoing a complex device implant. This helps explain why the odds of reporting severe pain were low among males. Anxiety is significantly more common among women,9 though predicting which patients may have high levels of anxiety is not easy, as models developed to explain pre-operative anxiety have a low predictive power.10 We believe this area warrants further research, in particular, with regard to patients with implantable defibrillators.
At present, it is not known what the causes of higher levels of pre-implant worry in this patient cohort are. We also do not understand whether higher levels of worry lead to any clinically meaningful sequelae, other than possible psychological distress. In patients with melanoma, for example, salivary cortisol peaks during lymph node biopsy when undertaken during local anaesthesia but not when undertaken during general anaesthesia, suggesting that procedural pain induces a physiological stress response.11 Cortisol is a measure of physiological stress and, in theory, should be beneficial in facilitating recovery from injury and surgery. However, it has been suggested that a stress response can be detrimental in modern surgical practice, particularly if prolonged.8 In the context of CIED, this is very unlikely to be the case given the limited area involved and the relatively short procedural time involved. However, cortisol measures have been associated with adverse psychological function following vascular surgery.12,13 Thus, it is possible that although high levels of worry prior to CIED implantation and subsequent experience of pain may not result in any physical harm or clinical deterioration, psychological morbidity may be adversely affected.
While we cannot attribute causality, we found that patients were much more likely to report high levels of worry if they were scheduled to have a defibrillator implanted. We also know that anxiety and depression are commonly reported in ICD recipients, particularly following shock delivery.14 Therefore, addressing pre-implant worry and anxiety may offer a strategy for improving intra-operative comfort and psychological wellbeing in the longer term, and this warrants future investigation.
Another finding from this study was that higher doses of lidocaine were also found to be weakly associated with higher levels of patient-reported severe pain. The most plausible explanation for this is that patients who experienced pain were given more lidocaine. Some clinicians buffer the acidic lidocaine with sodium bicarbonate in order to reduce the pain associated with the injection site. We do not buffer the lidocaine, and, therefore, an alternative, albeit less likely explanation, maybe that higher doses of injected anaesthetic may have caused more pain.
Operator factors also appear to influence the incidence of patient-reported severe pain. Operator 1 was associated with an increased risk of patient-reported pain and operator 2 was associated with a lower risk of patient-reported pain in univariate analysis. However, in a multi-variate analysis, the significance of operator 1 was lost and the benefit of operator 2 persisted. There are a number of potential explanations for why operator factors maybe important. Important operator differences include: training; experience of the operator; patient expectations of pain; operator perceptions of patient’s pain; local anaesthesia administration technique;15 use of buffering to reduce pain at local anaesthetic injection site; local aesthetic volume of distribution; time from local anaesthesia administration to incision; timing and dosing of intravenous anxiolytic and intravenous opioid (routine use versus only in response to patient pain). In a survey of 17 clinicians involved in CIED implants carried out at a regional educational meeting in March 2020, patient comfort was rated as the third most important aspect of the implant procedure (safety and infection prevention being rated higher). Clinicians also overestimated the amount of pain that patients experienced during CIED implantation, with 44% estimating that 10–20% of patients experienced severe pain and 38% estimating that 20–30% of patients experienced severe pain during CIED implantation. The implication is either that clinicians are aware of patient discomfort during CIED implantation but overestimate the prevalence, possibly because of recall bias, or that clinician’s recall may be accurate and that patients may be under-reporting their pain. This may be relevant as we are reporting patients’ recollections of pain during the procedure, rather than instantaneous reporting of pain throughout the procedure. Patient recollection of pain is influenced by the peak-end phenomenon whereby patients tend to recall the worst pain during an event rather than an average of the pain, and the memories can be significantly influenced by what happens at the end of the event.16 In this audit, we did not take this phenomenon into account and this is a limitation of this study. Pre-operative worry scores may be lowered by a sense of relief after having a safe smooth procedure, or the opposite if the procedure was unsuccessful, painful or complicated.
There are other limitations of this study. These include potential risk of bias caused by how the pain questionnaire was administered. The data were collected after the procedure and, therefore, recall of pre-implant worry may be influenced by any pain experienced during the implant. Also, numerous biases can occur while administering a questionnaire.17 We also use a non-standardised assessment of patient worry, a visual analogue scale, rather than a validated anxiety scale, such as the Hospital Anxiety Scale.18 Non-blinding of operators to the audit may have introduced bias in how operators delivered local anaesthesia and sedation. Furthermore, our audit recorded only 40.1% of the 1,483 CIED implants that were performed at our centre during the study period. This may affect the validity of our results. Explanations for a relatively low completeness include the fact that participating in the audit was voluntary not mandatory, and relied on staff to remember to conduct the audit survey and to have the time to administer the survey. We believe that non-participation in the audit occurred at random and that the sample is a representative sample of our current practice, but we acknowledge that we are not able to exclude that some missing cases did not occur at random and, thus, exclude the possibility of selection bias. However, the reader should bare these limitations in mind when interpreting the results and conclusions of this study.
An important variable that was not tested as a potential explanatory variable for increased pain was procedural time. A reasonable hypothesis would be that longer procedural times may be associated with increased pain as the local anaesthetic efficacy dwindles. In this study we did not record procedural times. This study was a pragmatic audit of prevalence of pain with an analysis of predictors of pain based on information that was available from chart review. Procedure time was not collected prospectively, nor is it available from chart review. It is our observation from routine clinical practice that it is likely that there is a bi-modal distribution of when a patient is likely to experience pain during a CIED implantation – early on (during incision and pocket fashioning) and late on – on closure or in particularly prolonged cases. The half-life of lidocaine is 1.5–2 hours. In our hospital, CRT cases rarely take longer than two hours. Overall 18/136 (13.2%) patients who had a de novo CRT experienced severe pain, which is higher than the overall study findings of 10.7%. The corollary of the notion that patients who undergo procedures that last longer are more likely to experience more pain is that patients who undergo procedures that are shorter should experience less pain. Our data do not support this because in the pulse generator group, 5/45 (11.1%) experienced severe pain, equivalent to, not less than, other groups. It is possible that this is a reflection of pain experience at the time of the incision. Therefore, while procedural time may be an important determinant in patient pain, it is likely that there are other important determinants. Future studies of CIED pain should record and investigate the importance of procedural time.
Last, the data were collected as part of an audit – first cycle followed by a brief pause in data collection (December 2017) followed by resumption of data collection for the second cycle. The main recommendation from the first cycle of the audit was for operators to be more cautious with regard to pain control during implants and to be more liberal with the use of intravenous anxiolytic medication. This intervention did not result in a meaningful difference in overall patient-reported pain (median pain score in the first cycle was 2 vs. 2 in second cycle; and mean pain score 3.0 vs. 3.2) and, thus, the data have been analysed as a whole rather than comparing the first to second cycle.
Conclusion
The majority of patients underwent CIED implantation with minimum stress and maximum comfort. An important minority reported at least severe pain during the procedure. High levels of pre-procedural worry, operator factors, gender and local anaesthetic dose were found to be important predictors of patient-reported pain. Interventions targeted at reducing pre- and peri-implant worry, particularly in women and those receiving ICDs, warrants further investigation as a means to reduce patient-reported pain during CIED implantation.
Key messages
- Research into evaluating pain during cardiac implantable electronic device (CIED) implantation is lacking despite data suggesting that pain occurs frequently and may not be benign
- We present data on patient-reported pain from 599 procedures, which shows that the majority of patients report no or mild pain, while approximately 10% of patients report severe pain
- We explore the predictors of pain and discuss the procedural and psychological factors that may be relevant in why some patients experience severe pain during CIED implantation
Conflicts of interest
DW reports grants from Boston Scientific ISR Lite Grant, personal fees from Novartis, personal fees from Astrazeneca, other from Medtronic, outside the submitted work. All other authors: none.
Funding
None.
Acknowledgements
Dr Jon Senior, Dr Boyang Liu, Ayman Jan for data collection and analysis.
Study approval
Ethical approval for this study was not sought as data were collected as part of a registered audit at Worcestershire Acute Hospitals NHS Trust.
References
1. Bode K, Breithardt O-A, Kreuzhuber M et al. Patient discomfort following catheter ablation and rhythm device surgery. EP Europace 2014;17:1129–35. https://doi.org/10.1093/europace/euu325
2. Boney O, Bell M, Bell N et al. Identifying research priorities in anaesthesia and perioperative care: final report of the joint National Institute of Academic Anaesthesia/James Lind Alliance Research Priority Setting Partnership. BMJ Open 2015;5:e010006. https://doi.org/10.1136/bmjopen-2015-010006
3. Fudim M, Dalgaard F, Al-Khatib SM et al. Future research prioritization in cardiac resynchronization therapy. Am Heart J 2020;223:48–58. https://doi.org/10.1016/j.ahj.2020.02.011
4. Kapa S, Chung M, Gopinathannair R et al. Year in review in cardiac electrophysiology. Circ Arrhythm Electrophysiol 2020;13:e008733. https://doi.org/10.1161/CIRCEP.120.008733
5. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res 2011;63(suppl 11):S240–S252. https://doi.org/10.1002/acr.20543
6. Biocic M, Vidosevic D, Boric M et al. Anesthesia and perioperative pain management during cardiac electronic device implantation. J Pain Res 2017;10:927–32. https://doi.org/10.2147/JPR.S132241
7. Lee JZ, Pasha AK, Glasgow AE et al. Postoperative opioid prescription patterns and new opioid refills following cardiac implantable electronic device procedures. Heart Rhythm 2019;16:1841–8. https://doi.org/10.1016/j.hrthm.2019.08.011
8. Hernández C, Díaz-Heredia J, Berraquero ML, Crespo P, Loza E, Ruiz Ibán M. Pre-operative predictive factors of post-operative pain in patients with hip or knee arthroplasty: a systematic review. Reumatol Clin 2015;11:361–80. https://doi.org/10.1016/j.reuma.2014.12.008
9. McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res 2011;45:1027–35. https://doi.org/10.1016/j.jpsychires.2011.03.006
10. Eberhart L, Aust H, Schuster M et al. Preoperative anxiety in adults – a cross-sectional study on specific fears and risk factors. BMC Psychiatry 2020;20:140. https://doi.org/10.1186/s12888-020-02552-w
11. Jansen P, Stoffels I, Müseler A-C et al. Salivary cortisol levels and anxiety in melanoma patients undergoing sentinel lymph node excision under local anesthesia versus general anesthesia: a prospective study. World J Surg Oncol 2020;18:53. https://doi.org/10.1186/s12957-020-01823-w
12. King AP, Abelson JL, Gholami B et al. Presurgical psychological and neuroendocrine predictors of psychiatric morbidity after major vascular surgery: a prospective longitudinal study. Psychosom Med 2015;77:993–1005. https://doi.org/10.1097/PSY.0000000000000235
13. Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The surgically induced stress response. J Parenter Enteral Nutr 2013;37(suppl 5):21S–29S. https://doi.org/10.1177/0148607113496117
14. de Ornelas Maia ACC, Soares-Filho G, Pereira V, Nardi AE, Silva AC. Psychiatric disorders and quality of life in patients with implantable cardioverter defibrillators: a systematic review. Prim Care Companion CNS Disord 2013;15:PCC.12r01456. https://doi.org/10.4088/PCC.12r01456
15. Quaba O, Huntley JS, Bahia H, McKeown DW. A users guide for reducing the pain of local anaesthetic administration. Emerg Med J 2005;22:188. https://doi.org/10.1136/emj.2003.012070
16. Kahneman D. Objective Happiness. Well-being: The Foundations of Hedonic Psychology. New York: Russell Sage Foundation, 1999; pp. 3–25.
17. Choi BCK, Pak AWP. A catalog of biases in questionnaires. Prev Chronic Dis 2005;2:A13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1323316/
18. Snaith RP, Zigmond AS. The hospital anxiety and depression scale. BMJ 1986;292:344. https://doi.org/10.1136/bmj.292.6516.344
