This article reviews some of the new concepts, new recommendations, along with changes to recommendations, in the diagnosis and investigation of heart failure (HF) in the European Society of Cardiology (ESC) 2021 Guidelines for the diagnosis and treatment of acute and chronic heart failure, and contrasts these with the 2016 version of the guidelines.
Introduction

The heart failure (HF) community has seen huge advances in the care of HF, and we see a turning point in the narrative of doom and gloom, which has traditionally been associated with HF – we see cause for optimism. We recognise the urgency of putting these advances to prompt use, as demonstrated by the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic HF.1 The 2021 updated guidelines make it clear that we have the means to diagnose HF early, to classify it more accurately, the tools to change the HF trajectory, and the duty and ability to intervene – and to do so early.
Diagnosis
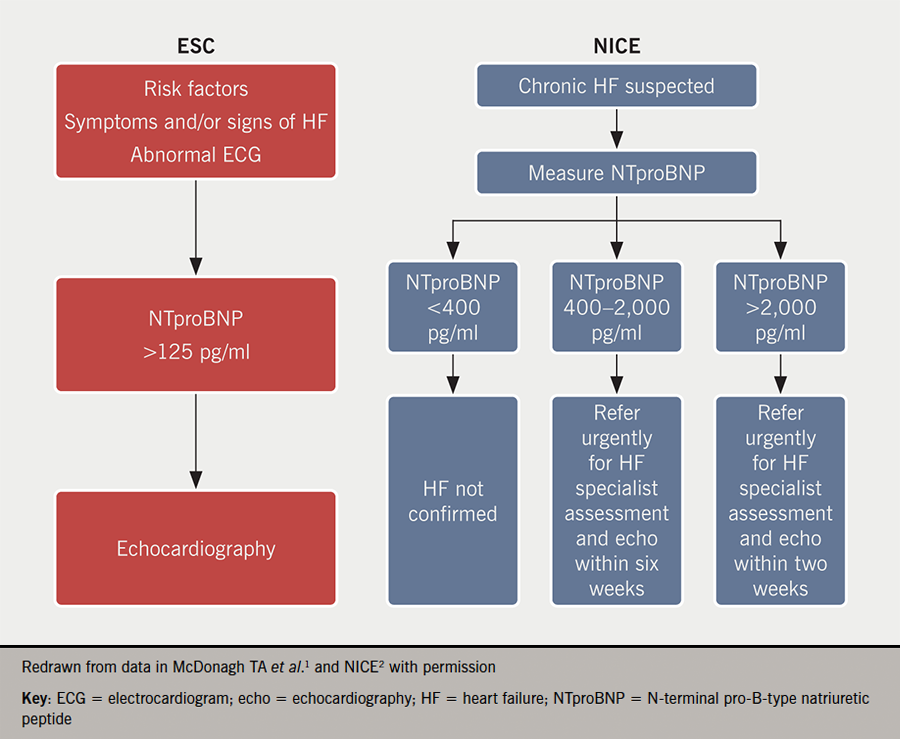
While the ESC diagnostic algorithm for HF is not new per se, it is worth reiterating and emphasising, that checking N-terminal pro-B-type natriuretic peptide (NTproBNP) levels and acting on them is the path to timely diagnosis. It allows for the early initiation of therapies to positively alter patient outcomes. The recommended cut-offs for NTproBNP are significantly lower in the ESC guidelines1 than in the National Institute for Health and Care Excellence (NICE) guidelines,2 as outlined in figure 1. They reflect that levels of NTproBNP below 125 pg/ml have excellent negative predictive value (0.94–0.98).
New concepts
These include:
- A change of the term ‘heart failure with mid-range ejection fraction’ to ‘heart failure with mildly reduced ejection fraction’ (HFmrEF).
- The nomenclature for HF with left ventricular ejection fraction (LVEF) of 41–49% has been revised to HF with mildly reduced EF (HFmEF).
HF with LVEF ≤40% remains HF with reduced EF (HFrEF), and HF with LVEF ≥50% remains HF with preserved EF (HFpEF).
For the diagnosis of HFmrEF, elevated natriuretic peptides plus other evidence of structural heart disease make the diagnosis more likely. But these are not mandatory if there is certainty on measurement of LVEF. The next article by Hardy and Kalra (pages S7–S12), will cover more on the addition of recommendations for the treatment of HFmrEF – patients with HFmrEF may benefit from similar therapies to those with HFrEF.
The guidelines now have a modified classification for acute HF, divided into four distinct types. Clinical presentations are mainly based on the presence of signs of congestion and/or peripheral hypoperfusion and require different treatments.3
- Acute decompensated heart failure (ADHF) is the most common form of AHF, accounting for 50–70% of presentations.4 ADHF usually occurs in patients with a history of HF and previous cardiac dysfunction across the spectrum of LVEF and may include right ventricular dysfunction. Distinct from the acute pulmonary oedema phenotype, it has a more gradual onset, and the main alteration is progressive fluid retention responsible for systemic congestion. Sometimes, the congestion is associated with hypoperfusion.4
- Acute pulmonary oedema related to lung congestion and clinical criteria for acute pulmonary oedema diagnosis include dyspnoea with orthopnoea, respiratory failure (hypoxaemia-hypercapnia), tachypnoea, >25 breaths/min, and increased work of breathing.5
- Isolated right ventricular (RV) failure is associated with increased RV and atrial pressure and systemic congestion. RV failure may also impair left ventricular (LV) filling, and ultimately reduce systemic cardiac output, through ventricular interdependence.6
- Cardiogenic shock is a syndrome due to primary cardiac dysfunction, resulting in inadequate cardiac output, leading to life threatening state of hypoperfusion which can result in multi-organ failure and death.7
New recommendations
New recommendations in diagnosis
Right heart catheterisation should be considered in patients where HF is thought to be due to constrictive pericarditis, restrictive cardiomyopathy, congenital heart disease, and high output states (Class IIa recommendation).
Right heart catheterisation may be considered in selected patients with HFpEF to confirm the diagnosis (Class IIb recommendation). The gold standard test for the diagnosis of HFpEF is invasive haemodynamic exercise testing. An invasively measured pulmonary capillary wedge pressure (PCWP) of ≥15 mmHg (at rest) or ≥25 mmHg (with exercise) or LV end-diastolic pressure ≥16 mmHg (at rest) is generally considered diagnostic.8 Recognising that invasive haemodynamic exercise testing is not available in many centres worldwide and has associated risks, the current guidelines do not mandate gold standard testing to make the diagnosis, but emphasise that the greater the number of objective non-invasive markers of raised LV filling pressures, the higher the probability the diagnosis of HFpEF (see table 1).
Table 1. Objective evidence used in the diagnosis of HFpEF
| Parameter | Threshold | Comments | ||
|---|---|---|---|---|
| LV mass index | >95 g/m2 (female) >115 g/m2 (male) |
The presence of concentric LV hypertrophy (LVH) is supportive, but its absence does not exclude a diagnosis of HFpEF | ||
| Relative wall thickness | >0.42 | Relative wall thickness (RWT) is increased in concentric LVH. Normal RWT is 0.32–0.42, measured using the equation RWT = IVSd + PWd / LVd | ||
| LA volume index | >34 ml/m2 (SR) >40 ml/m2 (AF) |
In the absence of AF or valvular disease, LA enlargement reflects chronically elevated LV filling pressure | ||
| E/e’ ratio at rest | >9 | Sensitivity 78%, specificity 59% for HFpEF by invasive exercise testing. Cut-off of 13 has lower sensitivity (46%) but higher specificity (86%)9 | ||
| NTpro BNP | >125 (SR), >365 (AF) pg/ml | Up to 20% of patients with invasively proven HFpEF have NPs below diagnostic thresholds, particularly in presence of obesity | ||
| PA systolic pressure/TR velocity at rest | >35 mmHg/>2.8 m/s | Sensitivity 54%, specificity 85% for presence of HFpEF by invasive exercise testing10 | ||
| Key: AF = atrial fibrillation; E/e’ = early filling velocity on transmitral Doppler/early relaxation velocity on tissue Doppler; HFpEF = heart failure with preserved ejection fraction; IVSd = interventricular septum thickness end diastole; LA = left atrium; LV = left ventricular; LVd = left ventricular diameter end diastole; NP = natriuretic peptides; NTproBNP = N-terminal pro-B-type natriuretic peptide; PA = pulmonary artery; PWd = posterior wall thickness end diastole; TR = tricuspid regurgitation; SR = sinus rhythm | ||||
New recommendations in monitoring
Non-invasive home telemonitoring (HTM) may be considered for patients with HF to reduce the risk of recurrent cardiovascular (CV) and HF hospitalisations and CV death (Class IIb recommendation).
Telemonitoring enables patients to provide their digital health information remotely to support and optimise their care. Data such as weight, heart rate, and blood pressure can be collected, stored in an electronic health record and used to guide changes to therapy. HTM allows for rapid access to care as needed, reduced patient inconvenience and travel costs, and minimises the frequency of clinic visits.11 The enforced cessation of face-to-face clinic visits in many countries during the COVID-19 pandemic has highlighted the potential advantages of HTM.12
New recommendations for HF and cancer care
There are two new recommendations in this area:
- It is recommended that cancer patients at increased risk for cardiotoxicity, defined by a history or risk factors of CV disease, previous cardiotoxicity or exposure to cardiotoxic agents, undergo CV evaluation before scheduled anticancer therapy, preferably by a cardiologist with experience/interest in cardio-oncology (Class I recommendation).
- A baseline CV risk assessment should be considered in all cancer patients scheduled to receive a cancer treatment with the potential to cause HF (Class IIa recommendation).
This is required because HF can occur in patients with cancer as a result of the interaction between the anticancer therapy, the cancer itself, and the patient’s own CV background (risk factors and coexisting CV disease).13 Several anticancer therapies may cause HF directly, through their cardiotoxic effects or, indirectly, through other mechanisms, such as myocarditis, ischaemia, systemic or pulmonary hypertension, arrhythmias or valve disease.14 HF, in turn, may affect cancer outcomes by depriving patients of effective anticancer therapies.15 Therefore, prevention of HF in patients with cancer receiving potentially cardiotoxic therapies requires careful patient assessment and management before, during, and after cancer therapy, preferably in the context of an integrated cardio-oncology service.13
Changes to recommendations
Changes to recommendations in diagnosis – three of note
Invasive coronary angiography may be considered in patients with HFrEF with an intermediate to high pre-test probability of coronary artery disease (CAD) and the presence of ischaemia in non-invasive stress tests (Class IIb recommendation [change from IIa], with removal of wording “who are considered suitable for potential coronary revascularisation”).
CT coronary angiography should be considered in patients with a low to intermediate pre-test probability of CAD or those with equivocal non-invasive stress tests to rule out coronary artery stenosis (Class IIa recommendation [change from IIb]).
Importantly, there have been key changes to the diagnosis of HFpEF. The guidelines now recommend a simplified diagnostic pathway, with three steps, for HFpEF, that include:
- Symptoms and signs of HF
- An LVEF ≥50%
- Objective evidence of cardiac structural and/or functional abnormalities consistent with the presence of LV diastolic dysfunction/ raised LV filling pressures, including raised natriuretic peptides (see table 1).
The guideline recommends a simplified approach to HFpEF diagnosis that distils the common major elements in prior diagnostic criteria, emphasising the most frequently used and widely available variables. Some of these, in particular:
- LA size (LA volume index >32 ml/m2)
- mitral E velocity >9 cm/s
- septal e’ velocity <9 cm/s
- E/e’ ratio >9
have been shown to be pivot points beyond which CV mortality risk is increased, underscoring their value.16 This recommendation is therefore consistent with the consensus document of the Heart Failure Association (HFA), but is, rather, a simplified approach.
Key changes to recommendations for the diagnosis of patients with cardiac amyloid
Cardiac amyloid is still an underdiagnosed cause of HF.17 The two most prevalent forms of cardiac amyloid are light chain immunoglobulin (AL) and transthyretin (ATTR) amyloidosis. ATTR includes wild-type (>90% of cases), and the hereditary type (<10% of cases). It is estimated that 6% to 16% of all patients with unexplained LVH or HFpEF at hospitalisation or severe aortic stenosis undergoing aortic valve replacement, aged above 65 years, may have wild typeTTR-CA.18 Based on a recent review, the latest guidelines recommend using major criteria for the suspicion of cardiac amyloid that include: age >65 years and HF, with LV wall thickness >12 mm at echocardiography.19 There are extensive resources within the guidelines to confirm the diagnosis, and include tables of ‘red flag’ findings.
Advice on cardiac imaging and electromyocardia biopsy (EMB) or extra-cardiac biopsy are also provided for the diagnosis of AL cardiac amyloid in patients with abnormal haematological tests. Technetium-labelled 99mTc-PYP or DPD or HMDP scintigraphy with planar and SPECT imaging has a specificity and positive predictive value for TTR cardiac amyloid of up to 100%.20 In contrast, CV magnetic resonance imaging (CMR) has a sensitivity and specificity of 85% and 92%, respectively.21 The hereditary form should be excluded by genetic testing. EMB is the gold standard for the diagnosis of TTR cardiac amyloid with nearly 100% sensitivity and specificity if specimens are collected from more than four multiple sites and tested for amyloid deposits by Congo red staining,21 but is not needed in the setting of grade 2–3 positive scintigraphy with SPECT.19
Lastly, there have been changes to the recommendations for gene testing in cardiomyopathies. While clinical history, laboratory tests, and imaging are the first-line investigations (with echocardiography as central in diagnosis and CMR providing more detailed morphological and prognostic information), testing for genetic mutations can add clinical and prognostic value. The prevalence of gene mutations may vary according to the phenotype or underlying cause. Gene mutations occur in up to 40% of dilated cardiomyopathy, 60% of hypertrophic cardiomyopathy, and 15% in chemotherapy-induced, alcoholic or peripartum cardiomyopathies.22 The prevalence of genetic mutations is also over 10% in non-familial dilated cardiomyopathy.22 Finding a pathogenic gene variant in a patient with cardiomyopathy allows better prediction of the disease outcome and progression, may contribute to the indications for device implantation and inform genetic counselling for families.
Of note, take heed, at the end of the guidelines, in sections 16 and 17, there are extremely useful summaries, entitled ‘Gaps in Evidence’, ‘What to do’ and ‘What not to do’ messages from the guidelines.
Conclusion
This 2021 updated ESC Guideline for diagnosis and treatment of HF1 indicates a radical shift – highlighting how we must accurately and promptly diagnose HF so that we can manage HF and encourage a patient-centric, tailored approach focused on improving patients’ quality of life and improving their clinical outcomes.
Key messages
- Use lower NTproBNP cut-offs for diagnosis – think heart failure (HF), and think it early
- HFmrEF is now termed HF with mildly reduced ejection fraction
- There is a simplified three-step HFpEF diagnostic pathway
Conflicts of interest
PC has received speaker fees for Astra Zeneca, Boehringer Ingelheim, Novartis, Pfizer, and Vifor.
Patricia Campbell
Consultant Cardiologist and Heart Failure Lead
Southern Health and Social Care Trust, Craigavon Area Hospital, 68 Lurgan Road, Portadown, BT63 5QQ
Articles in this supplement
Introduction
Drug therapy in heart failure – an update from the 2021 ESC heart failure guideline
Guidance on lifestyle, rehabilitation and devices in heart failure patients
References
1. McDonagh TA, Metra M, Adamo M et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368
2. National Institute for Health and Care Excellence. Chronic heart failure in adults: diagnosis and management. NG106, Published 12 September 2018. https://www.nice.org.uk/guidance/ng106
3. Nieminen MS, Brutsaert D, Dickstein K et al. The EuroHeart Survey Investigators, Heart Failure Association of the European Society of Cardiology. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 2006;27:2725–36. https://doi.org/10.1093/eurheartj/ehl193
4. Chioncel O, Mebazaa A, Maggioni AP et al. The ESC-EORP-HFA Heart Failure Long-Term Registry Investigators. Acute heart failure congestion and perfusion status – impact of the clinical classification on in-hospital and long-term outcomes: insights from the ESC-EORP-HFA heart failure long-term registry. Eur J Heart Fail 2019;21:1338–52. https://doi.org/10.1002/ejhf.1492
5. Masip J, Peacock WF, Price S et al. The Acute Heart Failure Study Group of the Acute Cardiovascular Care Association and the Committee on Acute Heart Failure of the Heart Failure Association of the European Society of Cardiology. Indications and practical approach to non-invasive ventilation in acute heart failure. Eur Heart J 2018;39:17–25. https://doi.org/10.1093/eurheartj/ehx580
6. Harjola VP, Mebazaa A, Celutkiene J et al. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail 2016;18:226–41. https://doi.org/10.1002/ejhf.478
7. Chioncel O, Parissis J, Mebazaa A et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:1315–41. https://doi.org/10.1002/ejhf.1922
8. Barandiaran Aizpurua A, Sanders-van Wijk S, Brunner-La Rocca HP et al. Validation of the HFA-PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail 2020;22:413–21. https://doi.org/10.1002/ejhf.1614
9. Lancellotti P, Galderisi M, Edvardsen T et al. Echo-Doppler estimation of left ventricular filling pressure: results of the multi-centre EACVI Euro-Filling study. Eur Heart J Cardiovasc Imaging 2017;18:961–8. https://doi.org/10.1093/ehjci/jex067
10. Pieske B, Tschope C, de Boer RA et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297–317. https://doi.org/10.1093/eurheartj/ehz641
11. Brahmbhatt DH, Cowie MR. Remote management of heart failure: an overview of telemonitoring technologies. Card Fail Rev 2019;5:86–92. https://doi.org/10.15420/cfr.2019.5.3
12. Cleland JG, Clark RA, Pellicori P, Inglis SC. Caring for people with heart failure and many other medical problems through and beyond the COVID-19 pandemic: the advantages of universal access to home telemonitoring. Eur J Heart Fail 2020;22:995–8. https://doi.org/10.1002/ejhf.1864
13. Zamorano JL, Gottfridsson C, Asteggiano R et al. The cancer patient and cardiology. Eur J Heart Fail 2020;22:2290–309. https://doi.org/10.1002/ejhf.1985
14. Zamorano JL, Lancellotti P, Rodriguez Munoz D et al. Authors/Task Force. Members, ESC Committee for Practice Guidelines, Document Reviewers. 2016. ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The TaskForce for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail 2017;19:9–42. https://doi.org/10.1093/eurheartj/ehw211
15. Ameri P, Canepa M, Anker MS et al., Heart Failure Association Cardio-Oncology Study Group of the European Society of Cardiology. Cancer diagnosis in patients with heart failure: epidemiology, clinical implications and gaps in knowledge. Eur J Heart Fail 2018;20:879–87. https://doi.org/10.1002/ejhf.1165
16. Playford D, Strange G, Celermajer DS et al., NEDA Investigators. Diastolic dysfunction and mortality in 436,360 men and women: the National Echo Database Australia (NEDA). Eur Heart J Cardiovasc Imaging 2021;22:505–15. https://doi.org/10.1093/ehjci/jeaa253
17. Martinez-Naharro A, Hawkins PN, Fontana M. Cardiac amyloidosis. Clin Med (Lond) 2018;18:s30–s35. https://doi.org/10.7861/clinmedicine.18-2-s30
18. Cavalcante JL, Rijal S, Abdelkarim I et al. Cardiac amyloidosis is prevalent in older patients with aortic stenosis and carries worse prognosis. J Cardiovasc Magn Reson 2017;19:98. https://doi.org/10.1186/s12968-017-0415-x
19. Garcia-Pavia P, Rapezzi C, Adler Y et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur J Heart Fail 2021;23:512–26. https://doi.org/10.1002/ejhf.2140
20. Gillmore JD, Maurer MS, Falk RH et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404–12. https://doi.org/10.1161/CIRCULATIONAHA.116.021612
21. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2872–91. https://doi.org/10.1016/j.jacc.2019.04.003
22. Hershberger RE, Givertz MM, Ho CY et al. Genetic evaluation of cardiomyopathy – a Heart Failure Society of America practice guideline. J Card Fail 2018;24:281–302. https://doi.org/10.1016/j.cardfail.2018.03.004
