Sodium-glucose cotransporter type 2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists are established glucose-lowering and weight-lowering agents used in the management of type 2 diabetes mellitus and obesity. Several recent clinical trials have provided evidence that these agents can decrease the risk of, and slow progression of, cardiovascular and renal diseases independently of their glucose-lowering and weight-lowering effects. In clinical trials and ‘real-world’ observational studies in people with and without diabetes, SGLT2 inhibitors have offered protection against heart failure and chronic kidney disease, while GLP-1 receptor agonists have been associated with reductions in atherosclerotic cardiovascular events and albuminuria. Based on this evidence, SGLT2 inhibitors and GLP-1 receptor agonists can now be considered for use beyond diabetes and obesity as new treatment options in the management of cardiorenal disease.
Introduction

Recent updates to guidelines for the management of type 2 diabetes (T2DM) have emphasised the importance of addressing cardiorenal risk and weight control, in conjunction with blood glucose regulation.1–4 All guidelines remain committed to lifestyle interventions (diet, physical activity and behavioural changes) as foundational therapy to be introduced at diagnosis, optimised and continued life-long. However, the progressive nature of T2DM typically requires the addition and dose-escalation of one or more blood glucose-lowering agents to achieve and maintain adequate glycaemic control.5 Several of the newer glucose-lowering agents have shown sufficient weight-reducing potency that they have been adopted for the treatment of obesity in people without diabetes.6,7 Also, some of these agents have shown cardioprotective and renoprotective effects that are independent of their glucose-lowering and weight-lowering effects.8 This narrative account summarises key evidence concerning the cardiorenal effects of glucose-lowering therapies, and notes opportunities for their use in people with and without T2DM or obesity.
Type 2 diabetes
Many different combinations of genetic and environmental factors conspire to impair insulin sensitivity (i.e. cause insulin resistance) and disrupt insulin secretion by pancreatic beta-cells, giving rise to the hyperglycaemia of T2DM (figure 1).9 Obesity and chronic low-grade inflammation aggravate insulin resistance during the prodromal (prediabetes) period, and the hyperinsulinaemia that initially compensates for insulin resistance exerts damaging sequelae on the vasculature and stress on pancreatic beta-cell function. The glucotoxic effects of persistent hyperglycaemia underpin the characteristic microvascular (chronic kidney disease [CKD] and retinopathies) and neuropathic complications of T2DM, while the broader metabolic disturbances (especially dyslipidaemia) plus insulin resistance contribute to the cardiovascular (CV) risk.10 The CV risk, in turn, adds to the renal risk, and vice versa.11 Accordingly, preference is now given to treatment strategies for T2DM that can directly address the cardiorenal risk, alongside the control of blood glucose and body weight.
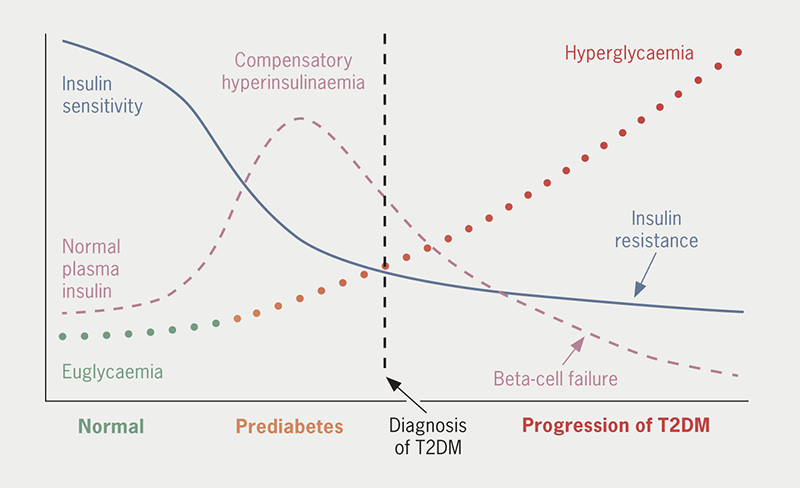
| The hyperglycaemia of T2DM is mainly caused by the combined impositions of declining insulin sensitivity (insulin resistance) and pancreatic beta-cell dysfunction, frequently aggravated by excess adiposity. Interactions of many genetic and environmental factors underlie the pathogenic process, creating the many different phenotypes of the disease. Insulin resistance is initially compensated by increased insulin secretion such that blood glucose is little changed, but ‘stress’ on the pancreatic beta-cells gradually interrupts normal insulin secretory function. In consequence, glucose tolerance becomes progressively impaired and eventually deteriorates into diabetes. Other gluco-regulatory disturbances contribute to the worsening hyperglycaemia, including increased glucagon secretion and a reduced effect of incretin hormones, notably glucagon-like peptide-1 (GLP-1). Associated metabolic alterations typically include dyslipidaemia (increases in triglycerides, very-low- and low-density lipoproteins and decreased high-density lipoproteins) and hypercoagulation. Beyond the well-recognised microvascular and macrovascular complications of T2DM there is increased risk of bone fractures, sarcopenia and cognitive disturbances. Based on reference 12. |
The main glucose-lowering agents that offer cardiorenal protective properties with weight restraint/loss are metformin, sodium-glucose cotransporter type 2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists (table 1).12 Other glucose-lowering agents continue to receive extensive and effective use in the management of T2DM, notably dipeptidyl-peptidase-4 (DPP4) inhibitors, pioglitazone and sulfonylureas, but these agents are more often used as second-line and third-line glucose-lowering therapies as they offer lesser or uncertain CV benefits (figure 2 and supplementary table 1).
Table 1. Key features of the main glucose-lowering agents used in the management of type 2 diabetes (T2DM)
| Class with examples |
a. Glucose-lowering efficacy^ b. Hypo risk^ c. Weight^ |
a. Cardiorenal effects b. Cautions and limitations c. Additional effects or comments |
| Oral | ||
| Biguanide Metformin (IR, SR/XR formulations) |
a. Efficacy high b. Hypo risk low c. Weight neutral |
a. Decrease CV mortality, increase survival, no evident effect on renal function but adequate renal function required for drug clearance (e.g. ≥30 ml/min/1.73 m2) b. Check renal function. Interrupt if using contrast media. Avoid in renal or liver impairment, sepsis, hypoxaemia, history of lactic acidosis or alcohol abuse. Rare risk of lactic acidosis. Check Vit B12 in long-term use c. Possible reduced risk of some cancers |
| Sulfonylureas Glibenclamide Gliclazide Gliclazide MR Glimepiride Glipizide Tolbutamide |
a. Efficacy high b. Hypo risk moderate c. Weight gain |
a. Clinically significant cardiorenal effects not established b. Avoid in renal or liver impairment depending on agent. Self-monitor blood glucose if driving or operating machinery c. Glucose-lowering efficacy wears-off with advancing beta-cell dysfunction |
| Meglitinides Nateglinide Repaglinide |
a. Efficacy intermediate b. Hypo risk moderate c. Weight gain |
a. Clinically significant cardiorenal effects not established b. Avoid in liver impairment. Self-monitor blood glucose if driving c. Take with main meals |
| DPP4 inhibitors Alogliptin Linagliptin Saxagliptin Sitagliptin Vildagliptin |
a. Efficacy intermediate-high b. Hypo risk low c. Weight neutral |
a. Clinically significant cardiorenal benefit not established b. Discontinue if acute pancreatitis. Adjust dose in renal impairment, except linagliptin c. Overall good safety profile |
| Thiazolidinedione Pioglitazone |
a. Efficacy high b. Hypo risk low c. Weight gain |
a. Possible reduced risk of atherosclerotic CV disease, notably stroke b. Slow onset of action. Risk of oedema, heart failure and bone fractures. Check liver enzymes |
| SGLT2 inhibitors Canagliflozin Dapagliflozin Empagliflozin Ertugliflozin |
a. Efficacy intermediate-high b. Hypo risk low c. Weight reduction |
a. Reduce onset/progression of heart failure and CKD b. Check for adequate renal function and hydration. Increased risk of genital and urinary infections, and euglycaemic ketosis c. Reduced blood pressure |
| Alpha-glucosidase inhibitors Acarbose |
a. Efficacy intermediate-low b. Hypo risk low c. Weight neutral |
a. Clinically significant cardiorenal benefit not established b. Avoid if gastrointestinal disorders c. Side effect of flatulence |
| Subcutaneous injection | ||
| GLP-1 receptor agonists Dulaglutide Exenatide BD Exenatide QW Liraglutide Lixisenatide Semaglutide Semaglutide oral Tirzepatide |
a. Efficacy high b. Hypo risk low c. Weight reduction |
a. Typically reduce albuminuria and some agents in class significantly reduce ASCVD and CV deaths b. Initial nausea, titrate as appropriate. Discontinue if acute pancreatitis c. Reduce blood pressure |
| Insulin Ultra-rapid acting: Fiasp, Lyumjev Rapid-acting: Aspart, Glulisine, Lispro Short-acting: Actrapid, Humulin S, Insuman Rapid Intermediate: Insulatard, Humulin I Long-acting: Degludec, Detemir, Glargine Biphasic (pre-mixed): Humalog, Humulin M3, Novomix |
a. Efficacy very high b. Hypo risk high c. Weight gain |
a. Clinically significant cardiorenal benefit not clear, although studies have shown acute effects that improve vasorelaxation and reduce components of atherogenesis and thrombus formation b. Select regimen consistent with patient lifestyle and needs. Requires appropriate lifestyle adjustments and glucose monitoring, especially if driving or operating machinery c. High risk of hypoglycaemia: carry glucose. Increase renal sodium reabsorption |
| ^ Based on ADA/EASD and AACE consensus statements.1,2 Key: ASCVD = atherosclerotic cardiovascular disease; CKD = chronic kidney disease; CV = cardiovascular; DPP4 = dipeptidyl peptidase-4; GLP-1 = glucagon-like peptide-1; hypo = hypoglycaemia; SGLT = sodium-glucose cotransporter; Vit = vitamin Based on reference 12. Some agents are not available in all countries, dosage forms and prescribing information listed in the summary of product characteristics may vary between countries. Additional agents (not listed here) have indications as glucose-lowering agents outside of the UK. Tirzepatide is a GLP-1/GIP dual receptor agonist. Fixed-dose combinations of oral agents (e.g. single tablet combinations of metformin with a DPP4 inhibitor, sulfonylurea, pioglitazone or SGLT2 inhibitor) and a fixed-ratio injectable combination of a GLP-1 receptor agonist with insulin are available. Pre-mixed insulins and biosimilar insulins are also available. Detemir is being discontinued during 2024. |
||
Figure 2. Summary diagram of metabolic and cardiorenal effects of glucose-lowering agents commonly used in the management of type 2 diabetes (T2DM)
| Cardiovascular outcome trials | |||||||
| Met | SGLT2i | GLP-1RA | DPP4i | SU | Pio | Insulin | |
| Diabetes | |||||||
| Glucose | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| Weight | ↔ | ↓ | ↓ | ↔ | ↑ | ↑ | ↑ |
| Hypos | ↔ | ↔ | ↔ | ↔ | ↑ | ↔ | ↑ |
| Cardiovascular | |||||||
| 3pt MACE | ↔ ↓ | ↔ ↓ | ↔ ↓ | ↔ | ↔ | ↔ ↓ | ↔ |
| CV deaths | ↔ ↓ | ↔ ↓ | ↔ ↓ | ↔ | ↔ | ↔ ↓ | ↔ |
| Heart failure | ↔ | ↓ | ↔ | ↔ | ↔ | ↔ ↑ | ↔ |
| Renal | |||||||
| Fall in eGFR | ↔ | ↓ | ↔ ↓ | ↔ | ↔ | ↔ | ↔ ↓ |
| Albuminuria | ↔ | ↓ | ↓ | ↔ | ↔ | ↔ | ↔ |
| Green = benefit; amber = neutral; red = adverse effect. ↑ increase; ↓ decrease; ↔ neutral. Note that it is difficult to compare insulin with other treatments in T2DM because individuals are typically given insulin because they are already in an advanced stage of the disease. Key: 3pt MACE = three-point major adverse cardiac events (cardiovascular deaths, non-fatal myocardial infarction and stroke); CV = cardiovascular; DPP4i = dipeptidylpeptidase-4 inhibitor; eGFR = estimated glomerular filtration rate; GLP-1RA = glucagon-like peptide-1 receptor agonist; Met = metformin; Pio = pioglitazone; SGLT2i = sodium-glucose cotransporter-2 inhibitor; SU = sulfonylurea |
|||||||
Supplementary table 1. Blood glucose-lowering agents used in the UK.*
| Class with examples |
Dose range mg/day (unless stated) |
a. Glucose-lowering efficacy^ b. Hypo risk^ c. Weight^ |
Mode of action | a. Cardiorenal effects b. Cautions and limitations c. Additional effects or comments |
| Oral | ||||
| Biguanide | a. Efficacy high b. Hypo risk low c. Weight neutral |
Counter insulin resistance ↓ hepatic glucose output ↑ peripheral glucose uptake ↑ splanchnic glucose cycling |
a. Decrease CV mortality, increase survival, no evident effect on renal function but adequate renal function required for drug clearance (e.g. ≥30 ml/min/1.73 m2) b. Check renal function. Interrupt if using contrast media. Avoid in renal or liver impairment, sepsis, hypoxaemia, history of lactic acidosis or alcohol abuse. Rare risk of lactic acidosis. Check Vit B12 in long-term use c. Possible reduced risk of some cancers |
|
| Metformin (IR, SR/XR formulations) | 500–3000 | |||
| Sulfonylureas | a. Efficacy high b. Hypo risk moderate c. Weight gain |
Initiate and potentiate insulin secretion (effect lasts 6–24 h depending on agent and dose) | a. Clinically significant cardiorenal effects not established b. Avoid in renal or liver impairment depending on agent. Self-monitor blood glucose if driving or operating machinery c. Glucose-lowering efficacy wears-off with advancing beta-cell dysfunction |
|
| Glibenclamide | 2.5–20 | |||
| Gliclazide | 40–320 | |||
| Gliclazide MR | 30–100 | |||
| Glimepiride | 1–6 | |||
| Glipizide | 2.5–20 | |||
| Tolbutamide | 500–3000 | |||
| Meglitinides | a. Efficacy intermediate b. Hypo risk moderate c. Weight gain |
Initiate and potentiate insulin secretion (rapid effect, typically lasts <6 h) | a. Clinically significant cardiorenal effects not established b. Avoid in liver impairment. Self-monitor blood glucose if driving c. Take with main meals |
|
| Nateglinide | 60–540 | |||
| Repaglinide | 0.5–16 | |||
| DPP4 inhibitors | a. Efficacy intermediate-high b. Hypo risk low c. Weight neutral |
Prolong circulating half-lives of incretin hormones such as GLP-1 | a. Clinically significant cardiorenal benefit not established b. Discontinue if acute pancreatitis. Adjust dose in renal impairment, except linagliptin c. Overall good safety profile |
|
| Alogliptin | 6.25–25 | |||
| Linagliptin | 5 | |||
| Saxagliptin | 2.5–5 | |||
| Sitagliptin | 25–100 | |||
| Vildagliptin | 50–100 | |||
| Thiazolidinedione | a. Efficacy high b. Hypo risk low c. Weight gain |
↑ insulin sensitivity mainly via activation of PPARγ | a. Possible reduced risk of atherosclerotic CV disease, notably stroke b. Slow onset of action. Risk of oedema, heart failure and bone fractures. Check liver enzymes |
|
| Pioglitazone | 15–45 | |||
| SGLT2 inhibitors | a. Efficacy intermediate-high b. Hypo risk low c. Weight reduction |
Inhibit renal SGLT2 to eliminate glucose via the urine | a. Reduce onset/progression of heart failure and CKD b. Check for adequate renal function and hydration. Increased risk of genital and urinary infections, and euglycaemic ketosis c. Reduced blood pressure |
|
| Canagliflozin | 100–300 | |||
| Dapagliflozin | 5–10 | |||
| Empagliflozin | 10–25 | |||
| Ertugliflozin | 5–15 | |||
| Alpha-glucosidase inhibitors | a. Efficacy intermediate-low b. Hypo risk low c. Weight neutral |
Slow carbohydrate digestion by competitive inhibition of intestinal glucosidases | a. Clinically significant cardiorenal benefit not established b. Avoid if gastrointestinal disorders c. Side effect of flatulence |
|
| Acarbose | 50–600 | |||
| Subcutaneous injection | ||||
| GLP-1 receptor agonists | a. Efficacy high b. Hypo risk low c. Weight reduction |
Activate GLP-1 receptors to potentiate prandial insulin secretion, ↓ prandial glucagon secretion, delay gastric emptying and exert satiety effect | a. Typically reduce albuminuria and some agents in class significantly reduce ASCVD and CV deaths b. Initial nausea, titrate as appropriate. Discontinue if acute pancreatitis c. Reduce blood pressure |
|
| Dulaglutide | 0.75–1.5 QW | |||
| Exenatide BD | 5–10 µg BD | |||
| Exenatide QW | 2 QW | |||
| Liraglutide | 0.6–1.8 OD | |||
| Lixisenatide | 10–20 µg OD | |||
| Semaglutide | 0.25–2 QW | |||
| Semaglutide oral | 3–14 mg/day | |||
| Tirzepatide | 2.5–15 QW | |||
| Insulin Ultra-rapid acting: Fiasp, Lyumjev Rapid-acting: Aspart, Glulisine, Lispro Short-acting: Actrapid, Humulin S, Insuman Rapid Intermediate: Insulatard, Humulin I Long-acting: Degludec, Detemir**, Glargine Biphasic (pre-mixed): Humalog, Humulin M3, Novomix |
Basal sc injections usually start at 0.1 or 0.2 units/kg body weight daily). Titrate up dose to achieve target glycaemic control. For MDI sc injections give ~30-50% as basal, and remainder divided between meals |
a. Efficacy very high b. Hypo risk high c. Weight gain |
↓ hepatic glucose output ↑ peripheral glucose uptake ↑ glucose metabolism ↓ lipolysis ↑ lipogenesis ↑ protein anabolism |
a. Clinically significant cardiorenal benefit not clear, although studies have shown acute effects that improve vasorelaxation and reduce components of atherogenesis and thrombus formation b. Select regimen consistent with patient lifestyle and needs. Requires appropriate lifestyle adjustments and glucose monitoring, especially if driving or operating machinery c. High risk of hypoglycaemia: carry glucose. Increase renal sodium reabsorption |
| Key: ASCVD = atherosclerotic cardiovascular disease; BD = twice daily; CKD = chronic kidney disease; CV = cardiovascular; DPP4 = dipeptidyl peptidase-4; GLP-1 = glucagon-like peptide-1; MDI = multiple daily insulin injections; OD = once daily; PPARγ = peroxisome proliferator-activated receptor-gamma; QW = once weekly; SGLT = sodium-glucose co-transporter; ↑ increase; ↓ decrease * Based on reference 12. Some agents are not available in all countries and may have different names, formulations and dosage strengths in different countries. Prescribing information in the summary of product characteristics may also vary between countries. Additional agents have indications as glucose-lowering agents outside of Europe, eg. colesevelam (bile sequestrant), bromocriptine (dopamine D2 receptor agonist) and pramlintide (amylin analogue taken as subcutaneous injections before meals) have an indication for diabetes in the USA. Tirzepatide is a GLP-1/GIP dual receptor agonist, administered by once weekly subcutaneous injection. Fixed-dose combinations of several oral agents are available (e.g. single tablet combinations of metformin with a DPP4 inhibitor, SU, pioglitazone or SGLT2 inhibitor), as well as a fixed-ratio injectable combination of a GLP-1 receptor agonist with insulin. Pre-mixed insulins and biosimilar insulins are also available. ** Detemir is being discontinued by Novo Nordisk during 2024. ^ Based on ADA/EASD and AACE consensus statements.1,2 |
||||
Metformin
Metformin is the most commonly used first-line glucose-lowering agent for people with T2DM.13 This agent is usually continued throughout the clinical course of T2DM, unless renal function falls below an estimated glomerular filtration rate (eGFR) of 30 ml/min/1.73 m2. Metformin counters insulin resistance and lowers blood glucose without precipitating overt hypoglycaemia, and can assist modest weight reduction and lowering of CV risk. As a long-established antidiabetic therapy (dating from 1957) with a respected safety profile, metformin has not been subjected to the recent large CV outcome trials (CVOTs) that have assessed major adverse cardiac events (MACE: CV deaths, non-fatal myocardial infarction [MI] and stroke) for more recently approved antidiabetic medications. However, extensive long-term use of metformin has provided substantial evidence to support its established use as a first-line therapy.14 It is also noted that the majority of patients with T2DM in the CVOTs were taking the newer agent as an add-on to metformin therapy.
Key evidence regarding metformin emerged in the United Kingdom Prospective Diabetes Study (UKPDS). This was a prospective randomised trial that found relative risk reductions in MI (by 39%), diabetes-related deaths (by 42%) and all-cause mortality (by 36%) after 10 years of treatment with metformin in overweight and obese newly diagnosed T2DM.15 These reductions were largely retained after 10 years of post-trial follow-up (by 33%, 30% and 27%, respectively, for MI, diabetes deaths and all-cause mortality).16 Other long-term prospective trials in T2DM have also noted fewer major CV events with metformin, and many long-term observational studies have corroborated these findings.17 For example, two meta-analyses of 16 and 40 observational studies found metformin reduced all-cause mortality by 56% and 33%, respectively.18,19 Two meta-analyses of nine and 11 observational studies involving T2DM patients with heart failure found that metformin was associated with reduced all-cause mortality by 20% and 22%, respectively.20,21
The CV effects of metformin appear to be largely independent of glycaemic and weight control. They have been attributed to modest improvements in endothelial-mediated vascular reactivity and decreased atherogenesis linked to reduced insulin resistance and an improved lipid profile.17 Regarding atherogenesis, metformin has been reported to reduce chronic inflammation, decrease endothelial production of attractant and adhesion molecules, reduce monocyte infiltration and foam cell formation, reduce oxidative stress and reduce hyperinsulinaemia.17 Additional, and often overlooked, effects of metformin include reduced hypercoagulation and improved thrombolysis associated with decreased platelet aggregation, altered fibrin polymerisation and reduced plasminogen-activator inhibitor-1 (PAI-1).22,23 Metformin has little effect on blood pressure beyond small reductions consistent with decreases in adiposity, and its impact on CV events has typically taken several years to emerge in the various studies. Also, it is reminded that metformin should not be continued in severe hypoxaemic disease.
The renal effects of metformin are often misrepresented. Metformin is eliminated unchanged in the urine, so use of metformin requires adequate renal function to prevent drug accumulation, which carries the risk (albeit rare) of lactic acidosis.14 Although metformin should be discontinued if renal function is severely impaired (eGFR <30 ml/min/1.73 m2), metformin does not harm the kidney and has been reported to reduce the risk or progression of kidney damage in diabetes.24,25 Metformin is best taken just before main meals with gradual dose titration to avoid/minimise gastrointestinal side effects, and it is noted that metformin has been reported to offer potential benefits against some cancers, age-related cognitive decline and certain infections.26
SGLT2 inhibitors
Since their introduction in 2012, SGLT2 inhibitors have become established blood glucose-lowering and body weight-lowering agents in the management of T2DM.12,27 They act as competitive inhibitors of SGLT2 in the proximal renal tubules, reducing glucose reabsorption from the renal filtrate and lowering blood glucose through increased glucosuria. The caloric deficit of the glucosuria (typically 60–100 g/day) enables the weight loss, while reductions in glucotoxicity and adiposity reduce insulin resistance and insulin concentrations. SGLT2 inhibitors do not cause overt hypoglycaemia because another sodium-glucose cotransporter (SGLT1) in the proximal tubules can reabsorb all of the glucose that remains in the filtrate at low glucose concentrations.
In addition to these metabolic effects, SGLT2 inhibitors afford cardiorenal protective benefits (figure 2).28–30 In a meta-analysis of large prospective CVOTs in T2DM patients, about two-thirds of whom had a history of atherosclerotic CV disease (ASCVD) (supplementary table 2), SGLT2 inhibitors reduced the risk of MACE by 10%, CV death by 15% and hospitalisation for heart failure (hHF) by 32%.30 While the reductions in MACE and CV death were statistically significant for those with ASCVD (11% and 17%, respectively), the numerical reductions in these events did not achieve statistical significance for those without ASCVD (6% and 5%, respectively). However, the reduction in hHF was highly significant for those with and without ASCVD (30% and 37%, respectively) and was evident within a few weeks of SGLT2 inhibitor initiation in individuals with a history of HF (supplementary table 3). Other analyses have confirmed these findings and reported all-cause mortality to be reduced by 13%.31 As noted above, the vast majority of patients in these trials were already taking metformin, but subanalyses indicate similar reductions in hHF occurred among those not taking metformin.32 Also, reductions in hHF were evident across a range of ejection fractions (HFrEF, HFmrEF, HFpEF) and in chronic kidney disease (CKD), and did not correlate with the extent of glucose-lowering or weight-lowering.33 This prompted further studies with two SGLT2 inhibitors (empagliflozin and dapagliflozin), which found reductions in hHF and CV death (by ~10–30%) in individuals without diabetes, and these agents now have broadened indications to include treatment of symptomatic chronic HF. Ongoing studies are assessing how the effects on HF are affected by ASCVD.29,34–36
Supplementary table 2. Cardiovascular outcome trials (CVOTs) with sodium-glucose co-transporter-2 (SGLT2) inhibitors in type 2 diabetes
| Trial | EMPA-REG | CANVAS | DECLARE | VERTIS | CREDENCE^ |
| Agent n |
Empagliflozin 7,020 |
Canagliflozin 10,142 |
Dapagliflozin 17,160 |
Ertugliflozin 8,246 |
Canagliflozin 4,401 |
| Type 2 diabetes % | 100 | 100 | 100 | 100 | 100 |
| Prior CV disease % | 100 | 65 | 41 | 100 | 50 |
| Prior heart failure % | 10 | 14 | 10 | 23 | 14 |
| Follow-up (median yr) | 3.1 | 2.4 | 4.2 | 3.0 | 2.6 |
| 3pt MACE | 0.86* 0.74, 0.99 |
0.86* 0.75, 0.97 |
0.93 0.84, 1.03 |
0.97 0.85, 1.11 |
0.80* 0.67, 0.95 |
| CV death | 0.62* 0.49, 0.77 |
0.87 0.72, 1.06 |
0.98 0.82, 1.17 |
0.92 0.77, 1.11 |
0.78 0.61, 1.00 |
| Non-fatal MI | 0.87 0.70, 1.09 |
0.85 0.69, 1.05 |
0.89 0.77, 1.01 |
1.00 0.86, 1.27 |
0.81 0.59, 1.10 |
| Non-fatal stroke | 1.24 0.92, 1.67 |
0.90 0.71, 1.15 |
1.01 0.84, 1.21 |
1.00 0.76, 1.32 |
0.80 0.56, 1.15 |
| Hospitalised HF | 0.65* 0.50, 0.85 |
0.67* 0.52, 0.87 |
0.73* 0.61, 0.88 |
0.70* 0.54, 0.90 |
0.61* 0.47, 0.80 |
| All cause death | 0.68* 0.57, 0.82 |
0.87 0.74, 1.01 |
0.93 0.82, 1.04 |
0.93 0.80, 1.08 |
0.83 0.68, 1.02 |
| Key: CV = cardiovascular; HF = heart failure; MACE = major adverse cardiovascular events; MI = myocardial infarction ^ CREDENCE patients had renal impairment defined as estimated glomerular filtration rate (eGFR) 30 to <90 ml/min/1.73 m2 and urinary albuminuria-creatinine ratio >300 to 5000 mg/g. * statistically significant. |
|||||
Supplementary table 3. Heart failure trials with sodium-glucose co-transporter-2 (SGLT2) inhibitors in people with or without type 2 diabetes
| Trial | DAPA-HF | EMPEROR | EMPEROR | DELIVER |
| Agent n |
Dapagliflozin 4,744 Reduced EF |
Empagliflozin 3,730 Reduced EF |
Empagliflozin 5,988 Preserved EF |
Dapagliflozin 6,263 Preserved EF |
| Type 2 diabetes % | 45 | 50 | 49 | 45 |
| LVEF % | 31 | 27 | 54 | 54 |
| Prior HF % | 100% HFrEF | 100% HFrEF | 100% HFpEF | 100% HFpEF |
| Follow-up (median yr) | 1.5 | 1.3 | 1.2 | 2.3 |
| Worse HF/CV death | 0.74* 0.64, 0.85 |
0.75* 0.65, 0.86 |
0.79* 0.69, 0.90 |
0.80* 0.71, 0.91 |
| CV death | 0.82* 0.69, 0.98 |
0.92 0.75, 1.12 |
0.91 0.76, 1.09 |
0.88 0.74, 1.05 |
| Hospitalised HF | 0.70* 0.59, 0.83 |
0.69* 0.59, 0.81 |
0.73* 0.61, 0.88 |
0.77* 0.67, 0.89 |
| All cause death | 0.83* 0.71, 0.97 |
0.92 0.77, 1.10 |
1.00 0.87, 1.15 |
0.94 0.83, 1.07 |
| * Statistically significant Key: CV = cardiovascular; EF = ejection fraction; HF = heart failure; HFrEF = heart failure with reduced ejection fraction, typically <40%; HFpEF = heart failure with preserved ejection fraction >40%; LVEF = left ventricular ejection fraction |
||||
The improved HF prognosis with use of SGLT2 inhibitors is ascribed, in part, to their antihypertensive action, which is associated with volume contraction due to the osmotic diuresis induced by the glucosuria. It is also suggested that the caloric deficit of glucosuria, which is compensated by mobilisation of fatty acids from adipose tissue, can improve myocardial energetics through increased supply of fatty acids and ketones.37,38 Direct effects of SGLT2 inhibitors on myocardial function, and reductions in inflammation and oxidative stress are further proposed mechanisms that reduce CV risk.37,38
A particularly advantageous feature of SGLT2 inhibitors is their protective effects against CKD.30,39 The onset and progression of CKD, as indicated by the decline in eGFR, is hastened in T2DM, but slowed during use of a SGLT2 inhibitor. This is typically accompanied by a reduction in albuminuria, and there are reports of reductions in some markers of kidney injury, renal inflammation and tubulointerstitial fibrosis.40 The nephro-protective effect of SGLT2 inhibitors has been noted at all stages of renal impairment and has prevented progression to end stage renal failure (supplementary table 4). For example, the CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) study in T2DM patients with an eGFR 30–90 ml/min/1.73 m2 and a urinary albumin–creatinine ratio (UACR) of 300–5000 mg/g, prospectively compared the SGLT2 inhibitor canagliflozin with placebo. Canagliflozin slowed the annual decline in eGFR by 1.5 ml/min/1.73 m2 (4.7 vs. 3.2 ml/min/1.73 m2 in the placebo and canagliflozin groups, respectively) and reduced UACR by ~30% (~900 vs. ~600 mg/g in the placebo and canagliflozin groups, respectively).41 Other studies have noted similar effects on renal function in people with and without diabetes,30,42,43 and various SGLT2 inhibitors are now approved to treat CKD in nondiabetic patients.
Supplementary table 4. Renal outcome data from cardiovascular outcome trials and renal trials with sodium-glucose cotransporter-2 (SGLT2) inhibitors in people with or without type 2 diabetes
| Trial | EMPA-REG | CANVAS | DECLARE | VERTIS | CREDENCE^ | DAPA-CKD^ | EMPA-KIDNEY^ |
| Agent n |
Empagliflozin 7,020 |
Canagliflozin 10,142 |
Dapagliflozin 17,160 |
Ertugliflozin 8,246 |
Canagliflozin 4,401 |
Dapagliflozin 4,304 |
Empagliflozin 6,609 |
| Type 2 diabetes (%) | 100 | 100 | 100 | 100 | 100 | 67 | 46 |
| eGFR ml/min/1.73 m2 ** | 74 | 76 | 85 | 76 | 56 | 43 | 37 |
| Microalbuminuria (%) | 29 | 22.7 | 23.4 | 30.2 | 11.4 | 10.3 | 28 |
| Macroalbuminuria (%) | 11 | 7.6 | 6.8 | 9.2 | 87.7 | 89.7 | 52 |
| Follow-up (median yr) | 3.1 | 2.4 | 4.2 | 3.0 | 2.6 | 2.4 | 2.0 |
| Slow decline in eGFR | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Renal composite*** varies between studies |
0.54* 0.40, 0.75 |
0.60* 0.47, 0.77 |
0.53* 0.43, 0.66 |
0.81 0.63, 1.04 |
0.70* 0.59, 0.82 |
0.56* 0.45, 0.68 |
0.72* 0.44, 0.82 |
| Decrease albuminuria | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| ^ CREDENCE, DAPA_CKD and EMPA-KIDNEY patients had renal impairment defined as estimated glomerular filtration rate (eGFR) 30 to <90 ml/min/1.73 m2 in CREDENCE, 25–75 ml/min/1.73 m2 in DAPA-CKD, and 20–45 (some albuminuric patients with eGFR 45–90) ml/min/1.73 m2 in EMPA-KIDNEY as well as a high proportion of patients with macroalbuminuria (urinary albuminuria-creatinine ratio >300 to 5000 mg/g). * statistically significant. **eGFR, estimated glomerular filtration rate by MDRD (EPI for Empagliflozin and Canagliflozin studies). Microalbuminuria, UACR 30–300 mg/g; macroalbuminuria >300 mg/g. *** Renal composite varies between studies: mostly 40% decrease in eGFR, end-stage / dialysis / transplant, renal death. In EMPA-REG, doubling serum creatinine, decrease eGFR <45 ml/min/1.73 m2, renal replacement, renal death and/or cardiovascular death. |
|||||||
The nephro-protective effect of SGLT2 inhibitors is mostly attributed to increased tubuloglomerular feedback, in which SGLT2 inhibition in the proximal tubule increases sodium passage along the nephron. Raised tubular sodium activates cells of the macula densa to promote the formation of adenosine, which diffuses locally and constricts adjacent afferent glomerular arterioles. The resulting decrease in intraglomerular pressure reduces hyperfiltration-mediated glomerular damage.44,45 Other effects of SGLT2 inhibitors on renal haemodynamics and energy substrate utilisation by the kidney have also been suggested.
For practical purposes, it is noted that eGFR will quickly fall by about 5 ml/min/1.73 m2 on initiation of a SGLT2 inhibitor, then level out and often gradually return over three to six months. Thus, although some SGLT2 inhibitors are approved to specifically treat CKD, their initiation is not recommended at an eGFR <20–25 ml/min/1.73 m2. It is useful to remind patients about possible urinary infections and to maintain adequate fluid intake.30,39
GLP-1 receptor agonists
GLP-1 receptor agonists (introduced in 2005) mimic the incretin effect, in which blood glucose concentrations are lowered by potentiating nutrient-induced insulin secretion.46 These agents also suppress glucagon secretion when glucose concentrations are raised, but not at low glucose concentrations, thus avoiding overt hypoglycaemia. Additionally, GLP-1 receptor agonists delay gastric emptying and exert a centrally mediated satiety effect, thereby facilitating weight loss.12,27 As peptide molecules, GLP-1 receptor agonists are expensive and administered by subcutaneous injection (although semaglutide is also available as an oral formulation), making them generally preferred as second-line or third-line agents for overweight/obese T2DM patients. Several members of the class exert particularly potent weight-loss effects, and high-dose formulations of these agents (liraglutide and semaglutide) and a related incretin-based peptide (tirzepatide) have received indications specifically to treat obesity.47,48
CVOTs conducted with GLP-1 receptor agonists in individuals with T2DM have produced numerical reductions in MACE, which have achieved statistical significance with some agents (figure 2 and supplementary table 5). Also, various components of MACE have been significantly reduced in these trials, but without a consistent pattern. In a meta-analysis of eight CVOTs, GLP-1 receptor agonists reduced the risk of MACE by 14%, CV death by 13%, all-cause mortality by 12% and hHF by 11%.49 Similar findings have been reported with other analyses that have included randomised and ‘real-world’ observational studies, and several studies have indicated significant reductions in non-fatal stroke and MI.50–52 The reductions in MACE and CV death achieved with GLP-1 receptor agonists in T2DM patients have been marginally greater among those with (rather than without) established CV disease, and appear to be largely independent of concurrent treatment with metformin or the extent of glucose-lowering and weight-lowering.51 Indeed, reductions in MACE have been observed during the use of GLP-1 receptor agonists in overweight/obese people without T2DM.48,53,54
Supplementary table 5. Cardiovascular outcome trials (CVOTs) with glucagon-like peptide-1 (GLP-1) receptor agonists in type 2 diabetes
| Trial | ELIXA | LEADER | SUSTAIN | PIONEER | EXSCEL | HARMONY | REWIND |
| Agent n |
Lixisenatide 6,068 |
Liraglutide 9,340 |
Semaglutide SC, QW 3,297 |
Semaglutide Oral, OD 3,183 |
Exenatide 14,752 |
Albiglutide 9,463 |
Dulaglutide 9,901 |
| Type 2 diabetes % | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Prior CV disease % | 100 | 82 | ~83 | ~84 | 73 | ~70 | 31 |
| Prior heart failure % | 22 | 14 | 23 | 12 | 16 | 20 | 8 |
| Follow-up (median yr) | 2.1 | 3.8 | 2.1 | 1.3 | 3.2 | 1.6 | 5.4 |
| 3pt MACE | 1.02^ 0.89, 1.17 |
0.87* 0.78, 0.97 |
0.74* 0.58, 0.95 |
0.79 0.57, 1.11 |
0.91 0.83, 1.00 |
0.78* 0.68, 0.90 |
0.88* 0.79, 0.99 |
| CV death | 0.98 0.78, 1.22 |
0.78* 0.66, 0.93 |
0.98 0.65, 1.48 |
0.49* 0.27, 0.92 |
0.88 0.76, 1.02 |
0.93 0.73, 1.90 |
0.91 0.78, 1.06 |
| Non-fatal MI | 1.03+ 0.87, 1.22 |
0.88 0.75, 1.03 |
0.74 0.51, 1.08 |
1.18 0.73, 1.19 |
0.97+ 0.85, 1.10 |
0.75+* 0.61, 0.90 |
0.96 0.79, 1.16 |
| Non-fatal stroke | 1.12+ 0.79, 1.58 |
0.89 0.72, 1.11 |
0.61* 0.38, 0.99 |
0.74 0.35, 1.57 |
0.85+ 0.70, 1.03 |
0.86+ 0.66, 1.14 |
0.76+ 0.61, 0.95 |
| Hospitalised HF | 0.96 0.75, 1.23 |
0.87 0.73, 1.05 |
1.11 0.77, 1.61 |
0.86 0.48, 1.55 |
0.94 0.78, 1.13 |
0.85++ 0.70, 1.04 |
0.93 0.77, 1.12 |
| All cause death | 0.94 0.78, 1.13 |
0.85* 0.74, 0.97 |
1.05 0.74, 1.50 |
0.51* 0.31, 0.84 |
0.86 0.77, 0.97 |
0.95 0.79, 1.16 |
0.90 0.80, 1.01 |
| Key: HF = heart failure; MACE = major adverse cardiovascular events; MI = myocardial infarction; OD = once daily; QW = once weekly; SC = subcutaneous * Statistically significant. ^ 4pt MACE. + may include some fatal cases |
|||||||
Receptors for GLP-1 are expressed by cardiomyocytes and endothelium, and GLP-1 receptor agonists have consistently reduced blood pressure in clinical trials. This has been attributed to a modest vasodilatory effect due, in part, to increased endothelial nitric oxide production, reduced angiotensin II production and, possibly, increased natriuresis.55 While this evidently helps to reduce atherogenesis, improves coronary perfusion and the myocardial microcirculation, GLP-1 receptor agonists may also act directly on myocardial cells to enhance nutrient metabolism and reduce oxidative stress.56
Assessment of renal function during the CVOTs, and other studies with GLP-1 receptor agonists, have consistently identified reductions in the onset and progression of albuminuria (supplementary table 6). Significant reductions in micro- and macro-albuminuria (typically UACR less than and more than 300 mg/g, respectively) have also been recorded in most of the studies with each of the various agents.51,57,58 For example, a meta-analysis of 27 studies found an overall 16% reduction in albuminuria in patients with T2DM, and efficacy was seen across the spectrum of CKD down to eGFR 15 ml/min/1.73 m2.57 Although undoubtedly assisted by improved control of blood glucose and body weight, the renal effects of GLP-1 receptor agonists were not closely correlated with the extent of glucose-lowering and weight-lowering, and appeared to be largely independent of the concurrent use of other glucose-lowering agents (except insulin). However, reductions in albuminuria were generally greater in individuals with a higher baseline systolic blood pressure (>130 mmHg), and it is these individuals who typically achieve a greater reduction of blood pressure during GLP-1 receptor agonist therapy. However, no association with any particular type of concurrent antihypertensive therapy was observed. Various composite measures of renal function, including changes in eGFR, progression to end-stage renal disease and cardiorenal mortality, have been reported in trials with GLP-1 receptor agonists. These have not shown consistent benefits, although some studies have noted a reduced rate of decline in eGFR.51,59 In the recently reported FLOW (Evaluate Renal Function with Semaglutide Once Weekly) trial, administration of semaglutide (1 mg weekly for 3.4 years) to T2DM patients with CKD (eGFR 25–75 ml/min/1.73 m2 and UACR >100 mg/g) reduced a renal composite of kidney failure (dialysis, transplantation or eGFR <15 ml/min/1.73 m2) or >50% decrease in eGFR or renal/CV death by 24%, and showed a reduced rate of decline in eGFR by 1.16 ml/min/1.73 m2.59
Supplementary table 6. Renal outcome data from cardiovascular outcome trials and renal trials with glucagon-like peptide-1 (GLP-1) receptor agonists in people with type 2 diabetes
| Trial | ELIXA | LEADER | SUSTAIN | PIONEER | EXSCEL | HARMONY | REWIND |
| Agent n |
Lixisenatide 6,068 |
Liraglutide 9,340 |
Semaglutide SC, QW 3,297 |
Semaglutide Oral, OD 3,183 |
Exenatide 14,752 |
Albiglutide 9,463 |
Dulaglutide 9,901 |
| Type 2 diabetes % | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| eGFR ml/min/1.73m2 | 76 | 80 | 76 | 74 | 76 | 76 | 76 |
| Albuminuria (%) | 25 | 11 | 33 | 22 | 34 | ||
| Follow-up (median yr) | 2.1 | 3.8 | 2.1 | 1.3 | 3.2 | 1.6 | 5.4 |
| Renal composite* varies between studies |
0.84 0.68–1.02 |
0.78 0.67–0.92 |
0.64 0.46–0.88 |
0.88 0.76–1.01 |
0.85 0.77–0.93 |
||
| New onset macroalbuminuria | 0.81 0.66–0.99 |
0.74 0.60–0.91 |
0.54 0.37–0.77 |
0.87 0.70–1.07 |
0.77 0.68–0.87 |
||
| End stage renal disease | 0.87 0.61–1.24 |
0.91 0.40–2.07 |
0.75 0.39–1.44 |
||||
| Key: OD = once daily; QW = once weekly; SC = subcutaneous Some studies did not measure all parameters or provide sufficient granular detail to fully populate the table. * Renal composite was not measured in all studies and varied between studies. LEADER and SUSTAIN assessed changes in albuminuria, serum creatinine, eGFR <45 ml/min/1.73 m2, need for dialysis and death from renal causes. EXSCEL assessed new macroalbuminuria, ≥40% eGFR decline, need for dialysis and death from renal causes, and REWIND assessed new macroalbuminuria, ≥30% eGFR decline, need for dialysis and death from renal causes. Data for renal composites are based on the analysis of Yu et al. Kidney Res Clin Pract 2022;41:136–49. |
|||||||
While the renal benefits of GLP-1 receptor agonists are primarily ascribed to reduced blood pressure and accompanying control of glycaemia and weight, it is noted that GLP-1 receptors are expressed by cells in the glomerulus and cortical segments of the renal tubules, and direct effects to reduce local inflammation, renal hypoxia and fibrosis have been suggested.60 In routine practice, once-weekly subcutaneously injected GLP-1 receptor agonists are now used more commonly than daily injections, and the dose is escalated slowly (as per product label) to minimise gastrointestinal side effects. Also, caution or discontinuation should be considered at eGFR 15–30 ml/min/1.73 m2, depending on the agent used.
Other glucose-lowering agents
The recently approved incretin-based dual agonist peptide, tirzepatide, activates receptors for GLP-1 and for glucose-dependent insulinotropic polypeptide (GIP), and has shown greater glucose-lowering and weight-lowering effects than current agonists of GLP-1 receptors alone.61 Initial evidence indicates that this dual agonist exerts similar or greater cardiorenal protective properties compared with agonists of GLP-1 receptors alone.48,62,63 Other dual receptor agonists for GIP/GLP-1 and glucagon/GLP-1 are advanced in development, as well as triple receptor agonists for GIP/GLP-1/glucagon and a combination of a GLP-1 receptor agonist with an amylin analogue.48 While the metabolic and cardiorenal effects of GLP-1 receptor agonists and SGLT2 inhibitors are well studied in combination with metformin, recent evidence suggests that the combination of a GLP-1 receptor agonist with a SGLT2 inhibitor can achieve greater decreases in blood glucose, body weight and blood pressure, with lower risk of MACE or serious renal events, than either alone.64–67
In current practice, there is still considerable reliance on other established glucose-lowering therapies in the management of T2DM, notably DPP4 inhibitors, sulfonylureas and pioglitazone.5 Many studies have affirmed the glucose-lowering efficacy of DPP4 inhibitors without causing overt hypoglycaemia, and often with some weight loss. DPP4 inhibitors have a good safety profile, but they have not shown significant cardiorenal benefits independently of glucose and weight control.12 Sulfonylureas are widely used in more severely hyperglycaemic individuals: they offer strong glucose-lowering efficacy for individuals who retain adequate beta-cell function. However, they are prone to cause weight gain and carry the risk of hypoglycaemia, and evidence for independent cardiorenal benefit remains inconclusive. Beyond its glucose-lowering effect, pioglitazone has been shown to improve the blood lipid profile, counter atherogenesis and reduce risk of stroke. However, weight gain, oedema, potential risk or aggravation of HF and other putative long-term safety concerns have limited usage.5,12
Impact on guidelines
As noted above, all guidelines for the management of T2DM emphasise the importance of lifestyle (diet, physical activity and behavioural changes) as foundational therapy, and present iterations of these guidelines have taken note of the cardiorenal effects of SGLT2 inhibitors and GLP-1 receptor agonists. Whereas metformin remains a preferred first-line pharmacotherapy for individuals without cardiorenal disease (‘glucocentric approach’), SGLT2 inhibitors and GLP-1 receptor agonists are now included as potential first-line pharmacotherapy for individuals who present with established cardiorenal disease or who are considered to be at particularly high risk (‘cardiorenal approach’) (figure 3).1–4 GLP-1 receptor agonists have shown greater benefits against ASCVD than SGLT2 inhibitors, whereas SGLT2 inhibitors have shown greater benefits against HF and CKD: thus, current guidelines are favouring early use of GLP-1 receptor agonists for patients with (or at particularly high risk of) ASCVD, while preference for early use of a SGLT2 inhibitor is given to patients with HF and/or CKD.1–4 Combination of two or more differently acting glucose-lowering agents is commonly required as T2DM progresses, and insulin may be required as part of combination therapy for severely hyperglycaemic and symptomatic patients. Avoidance of medication-induced hypoglycaemia and attention to weight control are important considerations throughout. Now that some glucose-lowering agents are indicated for use beyond the control of blood glucose and body weight, they provide new opportunities for use in the management of cardiovascular and renal disease in nondiabetic individuals.68–70 Moreover, expert groups have recently proposed interdisciplinary clinical recommendations that include SGLT2 inhibitors and GLP-1 receptor agonists within prevention and treatment strategies against a broad spectrum of conditions, including obesity, metabolic syndrome, prediabetes, diabetes, hypertension, ASCVD, CKD, and HF.71,72
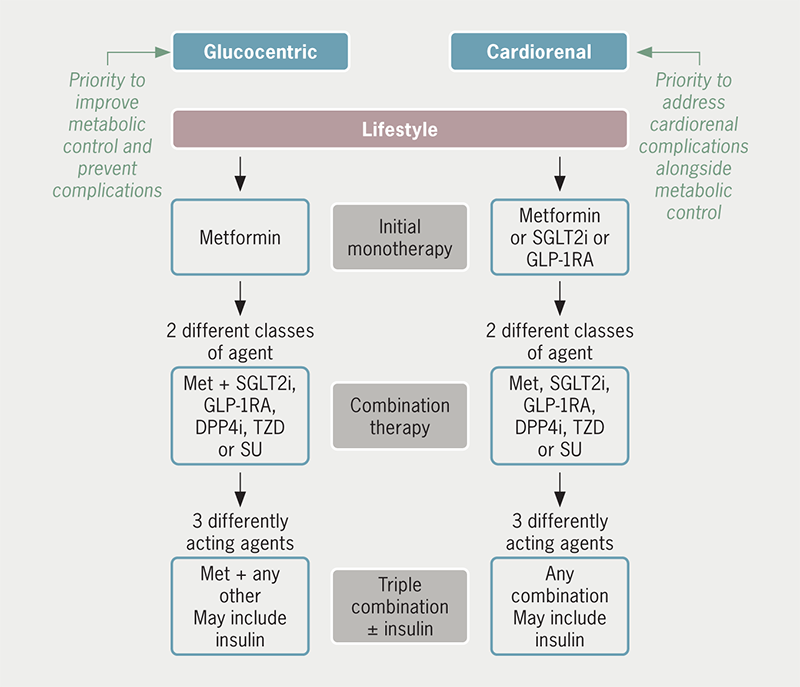
| Lifestyle (diet, physical activity and behavioural changes) is foundational therapy to be optimised and maintained throughout. Choice of initial pharmacotherapy may then be based upon whether priority is given to metabolic control (‘glucocentric approach’) or to addressing established cardiorenal disease or particularly high risk of cardiorenal disease (‘cardiorenal approach’). Key: DPP4i = dipeptidylpeptidase-4 inhibitor; GLP-1RA = glucagon-like peptide-1 receptor agonist; Met = metformin; SGLT2i = sodium-glucose cotransporter type 2 inhibitor; SU = sulfonylurea; TZD = thiazolidinedione (pioglitazone) |
Conclusion
Over the past two decades, the initial pharmacological management of hyperglycaemia in T2DM has been focused mostly on metformin, as it lowers blood glucose without causing overt hypoglycaemia, without weight gain and with long-term benefits to reduce cardiovascular events and mortality. SGLT2 inhibitors and GLP-1 receptor agonists are also now established glucose-lowering agents that avoid overt hypoglycaemia, and these agents offer the advantage of weight reduction plus protection against a range of cardiovascular and renal comorbidities and complications, including ASCVD, HF and CKD. Recent studies have shown that the cardiorenal effects of SGLT2 inhibitors and GLP-1 receptor agonists are substantially independent of their glucose-lowering and weight-lowering properties, and are evident in people without diabetes. Thus, SGLT2 inhibitors and GLP-1 receptor agonists present new therapeutic opportunities for cardiologists and nephrologists beyond diabetes and obesity.
Key messages
- Sodium-glucose cotransporter type 2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists are glucose-lowering and weight-lowering agents used in the management of type 2 diabetes mellitus and obesity
- SGLT2 inhibitors can also offer protection against the onset and progression of heart failure and chronic kidney disease in people with and without diabetes
- GLP-1 receptor agonists have been reported to reduce atherosclerotic cardiovascular events and albuminuria in people with and without diabetes
- SGLT2 inhibitors and GLP-1 receptor agonists may be considered for use beyond diabetes and obesity as treatment options in the management of cardiorenal disease
Conflicts of interest
None declared.
Funding
None.
Editors’ note
Supplementary tables are available online.
References
1. Davies MJ, Aroda VB, Collins BS et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022;45:2753–86. https://doi.org/10.2337/dci22-0034
2. Samson SL, Vellanki P, Blonde L et al. American Association of Clinical Endocrinology consensus statement: comprehensive type 2 diabetes management algorithm – 2023 update. Endocr Pract 2023;29:305–40. https://doi.org/10.1016/j.eprac.2023.02.001
3. Cosentino J, Grant PJ, Aboyans V et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J 2020;41:255–323. https://doi.org/10.1093/eurheartj/ehz486
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NG28. London: NICE, June 2022. Available from: https://www.nice.org.uk/guidance/ng28
5. American Diabetes Association Professional Practice Committee. Standards of care in diabetes 2024. Diabetes Care 2024;47(suppl 1):S5–S321. https://doi.org/10.2337/dc24-SREV
6. National Institute for Health and Care Excellence. Obesity: identification, assessment and management. CG189. London: NICE, July 2023. Available from: https://www.nice.org.uk/guidance/cg189
7. Cornier MA. A review of current guidelines for the treatment of obesity. Am J Manag Care 2022;28(suppl 15):S288–S296. https://doi.org/10.37765/ajmc.2022.89292
8. García-Carro C, Vergara A, Agraz I et al. The new era for reno-cardiovascular treatment in type 2 diabetes. J Clin Med 2019;8:864. https://doi.org/10.3390/jcm8060864
9. DeFronzo R, Ferrannini E, Groop L et al. Type 2 diabetes mellitus. Nat Rev Dis Primers 2015;1:15019. https://doi.org/10.1038/nrdp.2015.19
10. Lu Y, Wang W, Liu J et al. Vascular complications of diabetes: a narrative review. Medicine (Baltimore) 2023;102:e35285. https://doi.org/10.1097/MD.0000000000035285
11. Bell DSH, McGill JB, Jerkins T. Management of the ‘wicked’ combination of heart failure and chronic kidney disease in the patient with diabetes. Diabetes Obes Metab 2023;25;2795–804. https://doi.org/10.1111/dom.15181
12. Bailey CJ, Day C. Treatment of type 2 diabetes: future approaches. Br Med Bull 2018;126:123–37. https://doi.org/10.1093/brimed/ldy013
13. Bailey CJ. Metformin: historical overview. Diabetologia 2017;60:1566–76. https://doi.org/10.1007/s00125-017-4318-z
14. Bailey CJ. Metformin: therapeutic profile in the treatment of type 2 diabetes. Diabetes Obes Metab 2024;26(suppl 3):3–19. https://doi.org/10.1111/dom.15663
15. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–65. https://doi.org/10.1016/S0140-6736(98)07037-8
16. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. https://doi.org/10.1056/NEJMoa0806470
17. Schernthaner G, Brand K, Bailey CJ. Metformin and the heart: update on mechanisms of cardiovascular protection with special reference to comorbid type 2 diabetes and heart failure. Metabolism 2022;130:155–60. https://doi.org/10.1016/j.metabol.2022.155160
18. Zhang K, Yang W, Dai H, Deng Z. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: results from meta-analysis. Diabetes Res Clin Pract 2020;160:108001. https://doi.org/10.1016/j.diabres.2020.108001
19. Han Y, Xie H, Liu Y et al. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol 2019;18:96. https://doi.org/10.1186/s12933-019-0900-7
20. Eurich DT, Weir DL, Majumdar SR et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail 2013;6:395–402. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000162
21. Crowley MJ, Diamantidis CJ, McDuffie JR et al. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med 2017;166:191–200. https://doi.org/10.7326/M16-1901
22. Xin G, Wei Z, Ji C et al. Metformin uniquely prevents thrombosis by inhibiting platelet activation and mtDNA release. Sci Rep 2016;6:36222. https://doi.org/10.1038/srep36222
23. Dihoum A, Rena G, Pearson ER et al. Metformin: evidence from preclinical and clinical studies for potential novel applications in cardiovascular disease. Expert Opin Invest Drugs 2023;32:291–9. https://doi.org/10.1080/13543784.2023.2196010
24. Inzucchi SE, Lipska KJ, Mayo H et al. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014;312:2668–75. https://doi.org/10.1001/jama.2014.15298
25. Wang HH, Lin SH, Hung SY et al. Renal protective effect of metformin in type 2 diabetes patients. J Clin Endocrinol Metab 2024;online first. https://doi.org/10.1210/clinem/dgae477
26. Petrie J. Metformin beyond type 2 diabetes: emerging and potential new indications. Diabetes Obes Metab 2024;26(suppl 3):31–41. https://doi.org/10.1111/dom.15756
27. Tahrani A, Barnett A, Bailey C. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat Rev Endocrinol 2016;12:566–92. https://doi.org/10.1038/nrendo.2016.86
28. Vaduganathan M, Docherty KF, Claggett BL et al. SGLT2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet 2022;400:757–67. https://doi.org/10.1016/S0140-6736(22)01429-5
29. Kommu S. The role of SGLT2 inhibitors on heart failure outcomes in nondiabetic patients: a systematic review and meta-analysis of randomized controlled trials. J Cardiovasc Pharmacol 2024;83:158–66. https://doi.org/10.1097/FJC.0000000000001511
30. McGuire DK, Shih WJ, Cosentino F et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021;6:148–58. https://doi.org/10.1001/jamacardio.2020.4511
31. Giugliano D, Longo M, Scappaticcio L et al. SGLT-2 inhibitors and cardiorenal outcomes in patients with or without type 2 diabetes: a meta-analysis of 11 CVOTs. Cardiovasc Diabetol 2021;20:236. https://doi.org/10.1186/s12933-021-01430-3
32. Cahn A, Wiviott SD, Mosenzon O et al. Cardiorenal outcomes with dapagliflozin by baseline glucose-lowering agents: post hoc analyses from DECLARE-TIMI 58. Diabetes Obes Metab 2021;23:29–38. https://doi.org/10.1111/dom.14179
33. Usman MS, Bhatt DL, Hameed I et al. Effect of SGLT2 inhibitors on heart failure outcomes and cardiovascular death across the cardiometabolic disease spectrum: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2024;12:447–61. https://doi.org/10.1016/S2213-8587(24)00102-5
34. Rahman H, Khan SU, Lone AN et al. Sodium-glucose cotransporter-2 inhibitors and primary prevention of atherosclerotic cardiovascular disease: a meta-analysis of randomized trials and systematic review. J Am Heart Assoc 2023;12:e030578. https://doi.org/10.1161/JAHA.123.030578
35. Talha KM, Anker SD, Butler J. SGLT-2 inhibitors in heart failure: a review of current evidence. Int J Heart Fail 2023;5:82–90. https://doi.org/10.36628/ijhf.2022.0030
36. James S, Erlinge D, Storey RF et al. Dapagliflozin in myocardial infarction without diabetes or heart failure. NEJM Evid 2024;3:EVIDoa2300286. https://doi.org/10.1056/EVIDoa2300286
37. Dyck JRB, Sossalla S, Hamdani N et al. Cardiac mechanisms of the beneficial effects of SGLT2 inhibitors in heart failure: evidence for potential off-target effects. J Mol Cellular Cardiol 2022;167:17–31. https://doi.org/10.1016/j.yjmcc.2022.03.005
38. Packer M. SGLT2 inhibitors: role in protective reprogramming of cardiac nutrient transport and metabolism. Nat Rev Cardiol 2023;20:443–62. https://doi.org/10.1038/s41569-022-00824-4
39. Bailey CJ, Day C, Bellary S. Renal protection with SGLT2 inhibitors: effects in acute and chronic kidney disease. Curr Diab Rep 2022;22:39–52. https://doi.org/10.1007/s11892-021-01442-z
40. Liu H, Sridhar VS, Lovblom LE et al. Markers of kidney injury, inflammation, and fibrosis associated with ertugliflozin in patients with CKD and diabetes. Kidney Int Rep 2021;6:2095–104. https://doi.org/10.1016/j.ekir.2021.05.022
41. Perkovic V, Jardine MJ, Neal B et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–306. https://doi.org/10.1056/NEJMoa1811744
42. Baigent C, Emberson J, Haynes R et al. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 2022;400:1788–801. https://doi.org/10.1016/S0140-6736(22)02074-8
43. Podestà MA, Sabiu G, Galassi A et al. SGLT2 inhibitors in diabetic and non-diabetic chronic kidney disease. Biomedicines 2023;11:279. https://doi.org/10.3390/biomedicines11020279
44. Yau K, Cherney DZI, van Raalte DH, Wever BE. Kidney protective mechanisms of SGLT2 inhibitors: evidence for a hemodynamic effect. Kidney Int 2024;105;1168–72. https://doi.org/10.1016/j.kint.2024.03.019
45. Upadhyay A. SGLT2 inhibitors and kidney protection: mechanisms beyond tubuloglomerular feedback. Kidney 2024;5:771–82. https://doi.org/10.34067/KID.0000000000000425
46. Nauck MA, Müller FD. Incretin hormones and type 2 diabetes. Diabetologia 2023;66:1780–95. https://doi.org/10.1007/s00125-023-05956-x
47. Tschop M, Nogueiras R, Ahren B. Gut hormone-based pharmacology: novel formulations and future possibilities for metabolic disease therapy. Diabetologia 2023;66:1796–808. https://doi.org/10.1007/s00125-023-05929-0
48. Bailey CJ, Flatt PR, Conlon JM. Recent advances in peptide-based therapies for obesity and type 2 diabetes. Peptides 2024;173:171149. https://doi.org/10.1016/j.peptides.2024.171149
49. Sattar N, Lee MMY, Kristensen SL et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021;9:653–62. https://doi.org/10.1016/S2213-8587(21)00203-5
50. Bethel MA, Patel RA, Merrill P et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol 2018;6:105–13. https://doi.org/10.1016/S2213-8587(17)30412-6
51. Giugliano D, Scappaticcio L, Longo M et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol 2021;20:189. https://doi.org/10.1186/s12933-021-01366-8
52. Caruso I, Cignarelli A, Sorice GP et al. Cardiovascular and renal effectiveness of GLP-1 receptor agonists vs. other glucose-lowering drugs in type 2 diabetes: a systematic review and meta-analysis of real-world studies. Metabolites 2022;12:183. https://doi.org/10.3390/metabo12020183
53. Singh S, Garg A, Tantry US et al. Safety and efficacy of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese non-diabetic patients. Curr Probl Cardiol 2024;49:102403. https://doi.org/10.1016/j.cpcardiol.2024.102403
54. Kahn SE, Deanfield JE, Jeppesen OK et al. Effect of semaglutide on regression and progression of glycemia in people with overweight or obesity but without diabetes in the SELECT trial. Diabetes Care 2024;47:1350–9. https://doi.org/10.2337/dc24-0491
55. Pandey S, Mangmool S, Parichatikanond W. Multifaceted roles of GLP-1 and its analogs: a review on molecular mechanisms with a cardiotherapeutic perspective. Pharmaceuticals (Basel) 2023;16:836. https://doi.org/10.3390/ph16060836
56. Pahud de Mortanges A, Sinaci E, Salvador D Jr et al. GLP-1 receptor agonists and coronary arteries: from mechanisms to events. Front Pharmacol 2022;3:856111. https://doi.org/10.3389/fphar.2022.856111
57. Yuan D, Sharma H, Krishnan A et al. Effect of glucagon-like peptide 1 receptor agonists on albuminuria in adult patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab 2022;24:1869–81. https://doi.org/10.1111/dom.14776
58. Yu JH, Park SY, Lee DY et al. GLP-1 receptor agonists in diabetic kidney disease: current evidence and future directions. Kidney Res Clin Pract 2022;41:136–49. https://doi.org/10.23876/j.krcp.22.001
59. Perkovic V, Tuttle KR, Rossing P et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med 2024;391:109–21. https://doi.org/10.1056/NEJMoa2403347
60. Mosterd CM, Bjornstad P, van Raalte DH. Nephroprotective effects of GLP-1 receptor agonists: where do we stand? J Nephrol 2020;33:965–75. https://doi.org/10.1007/s40620-020-00738-9
61. De Block C, Bailey C, Wysham C et al. Tirzepatide for the treatment of adults with type 2 diabetes: an endocrine perspective. Diab Obes Metab 2023;25:3–17. https://doi.org/10.1111/dom.14831
62. Mima A, Lee R, Murakami A et al. Effects of incretin-based therapeutic agents including tirzepatide on renal outcomes in patients with type 2 diabetes: a systemic review and meta-analysis. Metabol Open 2023;17:100236. https://doi.org/10.1016/j.metop.2023.100236
63. Sattar N, McGuire DK, Pavo I et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med 2022;28:591–8. https://doi.org/10.1038/s41591-022-01707-4
64. Li C, Luo J, Jiang M, Wang K. The efficacy and safety of the combination therapy with GLP-1 receptor agonists and SGLT-2 inhibitors in type 2 diabetes mellitus: a systematic review and meta-analysis. Front Pharmacol 2022;13:838277. https://doi.org/10.3389/fphar.2022.838277
65. Williams N, Treves N, Yin H et al. Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: population based cohort study. BMJ 2024;385:e078242. https://doi.org/10.1136/bmj-2023-078242
66. Apperloo EM, Neuen B, Fletcher RA et al. Efficacy and safety of SGLT2 inhibitors with and without glucagon-like peptide 1 receptor agonists: a SMART-C collaborative meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol 2024;12:545–57. https://doi.org/10.1016/S2213-8587(24)00155-4
67. Mann JFE, Rossing P, Bakris G et al. Effects of semaglutide with and without concomitant SGLT2 inhibitor use in participants with type 2 diabetes and chronic kidney disease in the FLOW trial. Nat Med 2024;online first. https://doi.org/10.1038/s41591-024-03133-0
68. Novo Nordisk Limited. Semaglutide (Ozempic) summary of product characteristics. Available from: https://www.medicines.org.uk/emc/product/9750/smpc#gref
69. AstraZeneca UK Limited. Dapagliflozin (Forxiga) summary of product characteristics. Available from: https://www.medicines.org.uk/emc/product/7607/smpc#gref
70. Boehringer Ingelheim Limited. Empagliflozin (Jardiance) summary of product characteristics. Available from: https://www.medicines.org.uk/emc/product/5441/smpc#gref
71. Ndumele CE, Neeland IJ, Tuttle KR et al. A synopsis of the evidence for the science and clinical management of cardiovascular-kidney-metabolic (CKM) syndrome: a scientific statement from the American Heart Association. Circulation 2023;148:1636–64. https://doi.org/10.1161/CIR.0000000000001186
72. Handelsman Y, Butler J, Bakris GL et al. Early intervention and intensive management of patients with diabetes, cardiorenal, and metabolic diseases. J Diabetes Complications 2023;37:108389. https://doi.org/10.1016/j.jdiacomp.2022.108389
