Variable and sometimes ineffective drug treatment is not uncommon in the treatment of cardiovascular disease.(1) In most cases lack of patient compliance is the explanation but other reasons may also exist. In the case of aspirin, “low responsiveness” or “resistance” to aspirin in pharmacological terms would mean that the compound fails to reach its therapeutic goal, i.e. inhibition of platelet COX-1-dependent thromboxane formation. However, in vivo there might be also changes in platelet sensitivity or “residual” platelet activity independent of aspirin treatment, probably unrelated to the drug’s pharmacodynamic actions.(2)
Defining aspirin resistance
There is no universally accepted definition of aspirin resistance. In pharmacological terms, this means insufficient pharmacological inhibition of platelet cyclooxygenase-1 (COX-1)-derived thromboxane formation, with subsequent insufficient inhibition of platelet function, by standard antiplatelet doses of aspirin (75–300 mg/day).
Measuring aspirin resistance
In addition to the uncertainty regarding setting normal values and confidence intervals to define aspirin resistance, another major problem is the selection of appropriate methods for its determination. Measurement of inhibition of serum thromboxane quantitatively determines the pharmacological potency of aspirin to block the platelet COX-1 as seen from reducing the capacity of formation of an end product, i.e. thromboxane B2. However, serum thromboxane has no direct equivalent in terms of platelet aggregation or total body thromboxane generation.3 Further, serum thromboxane has no physiological or clinical correlate in vivo because the locally generated amounts of thromboxane at a site of thrombus formation in vivo are several orders of magnitude lower. In fact, in about 99% of cases aspirin does effectively block platelet COX-1, and thus pharmacological resistance does not account for clinical resistance.
In vitro platelet assays bypass the issue of COX-1-dependent thromboxane formation and have further significant limitations. Platelets are removed from the circulation, preventing the prothrombotic effects of shear stress and contact with blood cells and the vessel wall. Preactivation may occur and may be more marked in hyperreactive platelets. The assay usually involves stimulation by a single agonist whereas in vivo multiple agonists exert synergistic effects. Anticoagulants may modify platelet aggregation differently.4 Furthermore, there are no generally accepted normal ranges or assay standards and the reproducibility of assays is uncertain. Finally, there are important autocrine and paracrine functions of platelet-derived thromboxane, such as stimulation of platelet secretion or the thromboxane-dependent release of inflammatory mediators from platelets, such as sphingosine-1 phosphate.5 None of these secretory functions will be detected by measuring platelet aggregation.
The assessment of aspirin resistance in terms of platelet function is highly assay-dependent. In one well-designed double-blind study, the ASPECT trial,6 125 outpatients with coronary artery disease received three doses of aspirin (81, 162 or 325 mg/day) for four weeks each over a 12-week period and were tested for aspirin resistance by seven methods. The methods using arachidonic acid as an agonist suggested that the incidence of resistance to aspirin was low (0% to 6%) but others (including collagen-induced aggregation and the platelet function analyser) suggested that it might be as high as 27%. The efficacy of aspirin appeared to be dose-dependent when measured by some, but not all, methods. The ASPECT trial clearly shows also that repeated drug administration gives different results. It is also interesting to note that aspirin “resistance” as defined by urinary excretion of a thromboxane metabolite in this study “identifies” the highest percentage of aspirin “low responders”.6 However, in vitro platelet aggregation in platelet-rich plasma is much more sensitive to agonists such as ADP after anticoagulation by citrate than by antithrombins such as hirudin.4
Mechanisms of aspirin resistance

Although true pharmacological resistance to aspirin appears to be rare, two different forms of pharmacological variability of aspirin effects in platelets may be defined: drug-related and disease-related mechanisms. Table 1 gives an overview.
Drug-related mechanisms
Drug-related mechanisms may be pharmacokinetic or pharmacodynamic in nature. The former include low bioavailability or, more importantly, the interaction with non-steroidal anti-inflammatory drugs (NSAIDs). Insufficient bioavailability of aspirin has been described for 75 mg enteric-coated formulations.7 This agrees with the finding of Gurbel et al.6 that aspirin “resistance” occurs less frequently with increasing aspirin doses and repeated doses.6 The dosage of aspirin should be raised to 100 mg/day or more. The prevalence of a “true”, i.e. pharmacological “resistance” to aspirin is currently estimated to be about 1%.6,8,9
More important is the prevention of access of aspirin to its binding sites inside the COX‑1 channel by substrate competition. This type of drug interaction with the antiplatelet effects of aspirin was recognised for several anti-inflammatory drugs, including indomethacin and ibuprofen, many years ago.10-11 Interestingly, recent data also suggest similar interactions for the non-inflammatory pyrazoles, including dipyrone (metamizole)12 which is a frequently used and potent analgesic.13 Mechanistically, NSAIDs and pyrazoles will compete with arachidonic acid for access to the catalytic site inside the hydrophobic channel of COX-1 and might also prevent the initial binding of the salicylate moiety of aspirin. This type of interaction may become significant in patients at increased risk of myocardial infarction because of chronic diseases, such as rheumatoid arthritis.14
Pharmacodynamic reasons for aspirin resistance include COX-1 gene polymorphisms and alterations in COX-1 sensitivity to aspirin, which might be overcome by increasing aspirin doses. Several prothrombotic genetic variations relevant to antiplatelet effects of aspirin have been described,15 including those in COX-1.16 These heritable factors are assumed to contribute prominently to the variability in residual platelet activity which accounts for insensitivity to aspirin.17
Disease-related mechanisms
Clearly, treatment failure is caused much more often by lack of compliance and platelet hyperreactivity18 than by true pharmacological resistance to aspirin. These factors are frequently confused.19
Disease-related mechanisms of aspirin resistance include platelet hyperreactivity (as postulated to occur in patients with atherosclerosis), platelet stimulation by mechanisms insensitive to aspirin (such as adenosine diphosphate [ADP] agonism and shear stress), COX-2-dependent thromboxane formation (which will occur in inflammatory states such as atherosclerosis) and platelet sensitisation by isoprostanes (for example, in patients with diabetes) which cause platelet activation which is not sensitive to aspirin.20
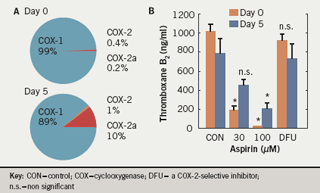
Fresh insights into possible mechanisms for aspirin “resistance” came from the discovery that platelets contain an immunoreactive COX-2.21 This was thought to offer an explanation for the different findings on aspirin resistance in vivo. We have shown that, in patients undergoing coronary artery bypass grafting (CABG) with extracorporeal circulation, inhibition of arachidonic acid-induced platelet aggregation by aspirin was reduced after five days; this corresponded with the expression of immunoreactive COX-2.13 However, the selective COX-2 inhibitor celecoxib did not block this effect. This apparent contradiction was resolved when a COX-2 isoform, COX-2a, was identified.22 COX-2a is normally present in very low concentrations (constituting 0.2% of total COX, compared with 0.4% for COX-2 and >99% for COX-1) but is induced in patients undergoing cardiac surgery so that it accounts for as much as 10% of total COX (compared with 1% for COX-2). This is associated with significant levels of thromboxane B2 even in the presence of aspirin (figure 1). The reduced responsiveness to aspirin in stroke patients may be overcome by increasing the aspirin dosage.23-24 It is not known whether an upregulation of an enzymatically active COX-2 occurs in other situations of increased platelet turnover, such as diabetes or essential thrombocythaemia, and how it compares to upregulation of COX-2a.
The role of inflammation
For many years, atherosclerosis has been recognised as an inflammatory condition. This implies that atherosclerosis, particularly in its advanced stages, might be associated with an upregulation of inflammatory genes and gene products, including COX-2 and prostaglandins.25 A subgroup analysis from the Heart Outcomes Prevention Evaluation (HOPE) study found a relationship between vascular outcomes and urinary excretion of the thromboxane metabolite 11-dehydro-thromboxane B2.26 It was suggested that urinary 11‑dehydro-thromboxane B2 might be an index of aspirin resistance but in reality it is more likely to reflect a systemic inflammatory state.27 Interestingly, the excretion of thromboxane metabolites correlates well with the measurement of platelet function,3 suggesting a significant contribution of non-platelet-COX-1-derived thromboxane to overall thromboxane production. Thus, chronic inflammatory conditions and/or advanced atherosclerosis, chronic heart and renal failure or hypertension might well be associated with an increased expression of COX-2, i.e. an aspirin-“resistant” form of PG-endoperoxide formation. In turn, this would provide endoperoxides for platelet thromboxane synthase not blocked by aspirin and will generate thromboxane A2 independent of the effective prevention of COX‑1-dependent thromboxane formation by aspirin.
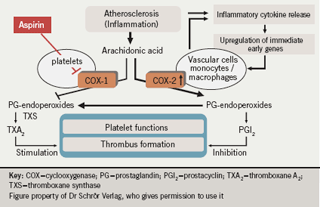
Figure 2 summarises this hypothesis. In the non-inflammatory state, arachidonic acid is metabolised first to prostaglandin endoperoxides and then to thromboxanes via a COX-1-dependent pathway and to prostaglandins via a COX-2 pathway. This balance between thromboxane and prostaglandin production is altered when an acute event causes the release of inflammatory cytokines. These upregulate COX-2 in inflammatory cells, such as monocytes and macrophages, but also in vascular cells including the endothelium and smooth muscle cells. COX-2 upregulation results in an increase in metabolism of arachidonic acid to prostaglandin endoperoxides, which are taken up by platelets and converted to thromboxane by their thromboxane synthase.
Aspirin blocks only the COX-1 pathway in this system, leaving the COX-2 pathway as a source of endoperoxides which are subsequently converted by platelets to thromboxanes. This model provides a mechanism for the diminished therapeutic response to aspirin prophylaxis and suggests that inflammation may have an important role in maintaining residual platelet activity. Patients who suffer from more advanced stages of atherosclerosis are likely to have more acute ischaemic/inflammatory events.
Summary
Insufficient pharmacological inhibition of platelet COX-1 by aspirin may exist but it is likely to be very rare (about 1%). The term aspirin “resistance” does not adequately explain treatment failures with low-dose aspirin which occur more often but have no direct pharmacological relationship to COX-1 inhibition by the drug. ‘Treatment resistance’ is a frequently used term for this phenomenon but of greater relevance in this context are patient adherence and ”residual” platelet reactivity. Aspirin “resistance”, however it is defined, is not a matter of concern28 and – excepting individual cases of aspirin intolerance – has no clinical consequences since no appropriate alternatives are available. Moreover, even if platelet function is incompletely inhibited, the drug may have effects on autocrine and paracrine functions of platelet-derived thromboxane. Thus, there is no reason to withhold aspirin alone or in dual antiplatelet therapy because of concerns regarding possible “resistance”.
Conflict of interest
KS has received research funding, honoraria for lectures or acted as a consultant to Bayer-Schering, Lilly/Daiichi Sankyo, Sanofi-Aventis and UCB.
References
- Mukherjee D, Topol EJ. Pharmacogenomics in cardiovascular diseases. Prog Cardiovasc Dis 2002;44:479–98.
- Cattaneo M. Laboratory detection of “aspirin resistance”; what test should we use (if any)? Eur Heart J 2007;28:1673–5.
- Santilli F, Rocca B, De Cristofaro R et al. Platelet cyclooxygenase inhibition by low-dose aspirin is not reflected consistently by platelet function assays: implications for aspirin “resistance”. J Am Coll Cardiol 2009;53:667–77.
- Bretschneider E, Glusa E, Schrör K. ADP-, PAF- and adrenaline-induced platelet aggregation and thromboxane formation are not affected by a thromboxane receptor antagonist at physiological external Ca++ concentrations. Thromb Res 1994;75:233–42.
- Böhm A, Ulrych T, Rosenkranz AC et al. Release of sphingosine-1-phosphate from platelets requires thromboxane synthesis and thromboxane receptor activation. Circulation 2009;120:S1080.
- Gurbel PA, Bliden KP, DiChiara J et al. Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation 2007;115:3156–64.
- Cox D, Maree AO, Dooley M et al. Effect of enteric coating on antiplatelet activity of low-dose aspirin in healthy volunteers. Stroke 2006;37:2153-8.
- Frelinger AL3rd, Furman MI, Linden MD et al. Residual arachidonic acid-induced platelet activation via an adenosine diphosphate-dependent but cyclooxygenase‑1- and cyclooxygenase-2-independent pathway: a 700-patient study of aspirin resistance. Circulation 2006;113:2888–96.
- Meen O, Brosstad F, Khiabani H, et al. No case of COX-1-related aspirin resistance found in 289 patients with symptoms of stable CHD remitted for coronary angiography. Scand J Clin Lab Invest 2008;68:185–91.
- Livio M, Del Maschio A, Cerletti C, de Gaetano G. Indomethacin prevents the long-lasting inhibitory effect of aspirin on human platelet cyclo-oxygenase activity. Prostaglandins 1982;23:787–96.
- Rao GH, Johnson GG, Reddy KR et al. Ibuprofen protects platelet cyclooxygenase from irreversible inhibition by aspirin. Arteriosclerosis 1983; 3:383–8.
- Hohlfeld T, Zimmermann N, Weber A-A et al. Pyrazolinone analgesics prevent the antiplatelet effect of aspirin and preserve human platelet thromboxane synthesis. J Thromb Haemost 2008;6:166–73.
- Zimmermann N, Wenk A, Kim U et al. Functional and biochemical evaluation of platelet aspirin resistance after coronary artery bypass surgery. Circulation 2003;108:542–7.
- Catella-Lawson F, Reilly MP, Kapoor SC et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 2001;345:1809–17.
- Cambria-Kiely JA, Gandhi PJ. Aspirin resistance and genetic polymorphism. J Thromb Thrombolysis 2002;14:51–8.
- Halushka MK, Walker MP, Halushka PV. Genetic variation in cyclooxygenase 1: effects on response to aspirin. Clin Pharmacol Ther 2003;73:122–30.
- Faraday N, Yanek LR, Mathias R et al. Heritability of platelet responsiveness to aspirin in activation pathways directly and indirectly related to cyclooxygenase-1. Circulation 2007;115:2490–6.
- Cattaneo M. Laboratory detection of “aspirin resistance”; what test should we use (if any)? Eur Heart J 2007;28:1673–5.
- Hennekens CH, Schrör K, Weisman S et al. Terms and conditions. Semantic complexity and aspirin resistance. Circulation 2004;110:1706–08.
- Cipollone F, Ciabattoni G, Patrignani P et al. Oxidant stress and aspirin-insensitive thromboxane biosynthesis in severe unstable angina. Circulation 2000;102:1007–13.
- Weber AA, Zimmermann KC, Meyer-Kirchrath J et al. Cyclooxygenase-2 in human platelets as a possible factor in aspirin resistance. Lancet 1999;353:900.
- Censarek P, Steger G, Paolini C et al. Alternative splicing of platelet cyclooxygenase-2 mRNA in patients after coronary artery bypass grafting. Thromb Haemost 2007;98:1309–15.
- Helgason CM, Bolin KM, Hoff JA et al. Development of ASA resistance in persons with previous ischemic stroke. Stroke 1994;25:2331–6.
- Hohlfeld T, Weber A-A, Junghans U et al. Variable platelet response to aspirin in patients with ischemic stroke. Cerebrovasc Dis 2007;24:43–50.
- Belton O, Byrne D, Kearney D et al. Cyclooxygenase-1 and –2-dependent prostacyclin formation in patients with atherosclerosis. Circulation 2000;102:840–5.
- Eikelboom JW, Hirsh J, Weitz JI et al. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation 2002;105:1650–5.
- Schrör K. Acetylsalicylic Acid. Wiley VCH: Weinheim, 2009.
- Schrör K, Hohlfeld T, Weber AA. Aspirin resistance – does it clinically matter? Clin Res Cardiol 2006;95:505–10.
