Acarbose is an alpha-glucosidase inhibitor acting in the gastrointestinal tract producing modest reductions in postprandial hyperglycaemia, with negligible risk of hypoglycaemia and weight gain. In a subgroup of the United Kingdom Prospective Diabetes Study (UKPDS), acarbose showed glycaemic benefits irrespective of the type of concomitant therapy. Acarbose was shown to produce a significant reduction in the progression to diabetes in patients with impaired glucose tolerance in the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) trial, and in a post-hoc analysis of STOP-NIDDM a reduction in cardiovascular events was observed. Gastrointestinal side effects are the main limiting factor in clinical practice, leading to high rates of non-compliance and discontinuation.
Introduction
Type 2 diabetes mellitus (T2DM) is evolving into a pandemic with major concerns about its long-term management. The list of oral and injectable therapies for type 2 diabetes is expanding at a brisk pace, with promising newer classes of drugs in the pipeline. Acarbose came into clinical use in the 1990s as an alternative oral antidiabetic drug to metformin and sulphonylureas. Despite its glycaemic, and possible cardiovascular benefits, its high rate of gastrointestinal side effects has limited its use in the UK. The results of two major ongoing trials involving acarbose in non-diabetic subjects with established cardiovascular disease may increase our understanding of its possible cardiovascular benefits.
Pharmacology
Acarbose is an oligosaccharide derived from the Actinoplanes strain of fungi. The mechanism of action is predominantly through competitive, reversible inhibition of intestinal brush border alpha-glucosidase, with a weaker effect on pancreatic alpha-amylase. The overall effect is the reduction in production and absorption of monosaccharides in the small intestine (figure 1). The activity of alpha-glucosidase varies between individuals with the dosage of acarbose adjusted according to clinical response and side effects. The duration of action is up to six hours. It is effective when ingested at the onset of a meal and the advice is to take it at the first bite of the meal. The delay in the absorption of carbohydrates leads to a reduction in postprandial hyperglycaemia. Additionally, acarbose produces a mild reduction in fasting hyperglycaemia. It reduces both fasting and postprandial insulin levels. Hypoglycaemia occurring during treatment with acarbose must be treated with glucose only, and not sucrose, as a consequence of its mechanism of action.
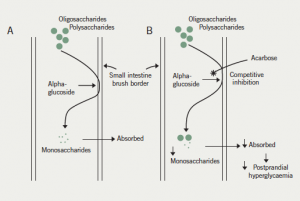
Acarbose can be used as an adjunct to diet and exercise as monotherapy when other oral antidiabetic agents are contraindicated, or in any combination of oral antidiabetic drugs and insulin in the management of type 2 diabetes mellitus. There is a 0.4–1% reduction in glycosylated haemoglobin (HbA1c) with acarbose monotherapy, and up to a 0.65% reduction with combination therapy with other antidiabetic medications. Triglycerides and low-/high-density lipoprotein (LDL/HDL) cholesterol ratio have decreased with acarbose in some studies. Acarbose monotherapy is not associated with any significant change in weight.
The drug has a local effect in the small intestine with less than 2% of the drug absorbed unchanged. Acarbose is cleaved in the large intestine, and partly absorbed and excreted through the kidneys. There is no treatment failure on prolonged use and the efficacy and side effects improve with the duration of use.
It is especially used in reducing postprandial hyperglycaemia. It is used in situations where there is a discrepancy between the blood glucose values obtained on self-monitoring of blood glucose values and the HbA1c. A mildly elevated fasting glucose and a disproportionately high HbA1c suggests postprandial hyperglycaemia. It also reduces reactive hypoglycaemia by delaying the glucose absorption peak. The gastrointestinal side effects are favourable in liver cirrhosis in reducing encephalopathy.1 Its side effects can be utilised beneficially in the elderly, where constipation and hypoglycaemia can be countered while improving the glycaemic profile.
It is approved for use in impaired glucose tolerance to delay or halt the progression to type 2 diabetes. Acarbose has been shown to reduce postprandial glucose levels in insulin treated diabetes, including type 1 diabetes when it is used as an adjunct to insulin, but the reduction in HbA1c noted in this group has been very marginal and not consistent.2
Flatulence is very common, and abdominal cramps and diarrhoea, as well as dyspepsia and nausea, are common side effects that are proportional to the amount of carbohydrate intake. The gastrointestinal side effects are secondary to the fermentation of undigested carbohydrates in the colon producing gaseous by-products.2 Acarbose should be started at a low dose of 50 mg once daily, followed by slow upward dose titration every few weeks to reduce gastrointestinal adverse effects. The dose range is 50 mg once daily to 200 mg thrice daily. A diet high in complex carbohydrates and low in simple sugars reduces side effects. Hepatic enzyme elevation is uncommon, but has been noted in some cases with high doses.
The drug is contraindicated in pregnancy, lactation and those below 18 years of age. It is contraindicated in inflammatory bowel disease, colonic ulceration, subacute and total intestinal obstruction, malabsorption states, severe renal impairment with creatinine clearance below 25 ml/min and large gastrointestinal hernias.
Neomycin and cholestyramine enhance the efficacy and side effects of acarbose, and digoxin levels can be altered and may need monitoring.
Trials of safety and efficacy
Evidence for improved glycaemic control
Acarbose is a safe drug and the beneficial effects of acarbose in improving glycaemic control have been shown in several studies. The Essen3 and Essen-II4 studies were performed by doctors working for the manufacturer and suggested that acarbose 100 mg three times a day was equipotent to glibenclamide 3.5 mg twice daily and metformin 850 mg twice daily, respectively. Slight improvements in lipid profiles were noted with acarbose in both these studies.
One of the substudies of the United Kingdom Prospective Diabetes Study (UKPDS 44) compared 1,946 patients with type 2 diabetes, who were equally randomised to either acarbose or a matching placebo.5 Altogether, 14% of patients were treated with diet alone, 52% with monotherapy and 34% with combined therapy. The dose of acarbose was titrated to a maximum of 100 mg three times a day. Patients were monitored four-monthly for three years. Acarbose appeared to be equally efficacious when given either alone or with oral antidiabetic agents or insulin or their combination, producing an improvement of 0.5% in HbA1c in patients remaining on their allocated therapy. A significantly greater number of patients in the acarbose group were noncompliant compared with the placebo group, mainly due to flatulence and diarrhoea. At the end of three years, only 39% of subjects remained on acarbose, compared with 58% of subjects on placebo. The duration of this substudy was not long enough to comment on any possible reduction in diabetic complications.
The Precose Resolution of Optimal Titration to Enhance Current Therapies (PROTECT) study6 was a post-marketing study involving 6,142 patients showing reasonable safety and efficacy, with a mean reduction in HbA1c of 0.66%.
National Institute for Health and Clinical Excellence (NICE) guidelines suggest using acarbose as monotherapy when other oral antidiabetic medications are not able to be used, because of its lower glucose-lowering efficacy, higher dropout rate due to intolerance and higher cost in comparison with well-established therapies. A similar view is expressed in the Scottish Intercollegiate Guidelines Network (SIGN) guidelines.
Evidence for cardiovascular benefit
While peripheral insulin resistance is the main aetiology for fasting hyperglycaemia, increased hepatic glucose output and delayed insulin release are responsible for impaired glucose tolerance. Impaired glucose tolerance is more strongly associated with negative cardiovascular outcomes than fasting hyperglycaemia. There are many studies pointing to postprandial glycaemia as an independent risk factor for cardiovascular diseases.
The Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) trial7 was a multi-centre, double-blind, placebo-controlled trial involving 1,429 individuals with impaired glucose tolerance. Altogether, 49% were men and 51% women with a mean (standard deviation [SD]) age of 54.5 (7.9) years and body mass index of 30.9 (4.2) kg/m2. Patients were randomised to receive either placebo or acarbose 100 mg three times a day and were followed-up for a mean duration of 3.3 years. There was a 2.5% absolute risk reduction and a 36% relative risk reduction of progression to diabetes, which was the primary end point of the study.7 In a post-hoc analysis a 34% relative risk reduction in the development of new cases of hypertension (hazard ratio [HR] 0.66; 95% confidence interval [CI] 0.49–0.89; p=0.006), and a 49% relative risk reduction in cardiovascular events (HR 0.51; 95% CI 0.28–0.95; p=0.03) were observed.8 Most of the reductions were in myocardial infarctions. In a small subgroup analysis from STOP-NIDDM there was also a reduction in progression of carotid intima-media thickness with the use of acarbose.9 The results of STOP-NIDDM, and in particular the possible cardiovascular benefits, are controversial and a detailed critical review found several series flaws in the STOP-NIDDM study, especially selection bias, inadequate blinding, bias in data analysis and reporting, and potential sponsoring bias.10
A meta-analysis of seven randomised, double-blind, placebo-controlled studies of acarbose in type 2 diabetes by Hanefeld et al.11 has shown that acarbose treatment significantly reduced the risk of myocardial infarction (HR 0.36; 95% CI 0.16–0.80; p=0.0120) and any cardiovascular event (HR 0.65; 95% CI 0.48–0.88; p=0.0061). There were also improvements in glycaemic control, triglyceride levels, body weight and systolic blood pressure. These factors are all associated with increased risk of cardiovascular events in type 2 diabetes. This meta-analysis is also controversial as much of the data were unpublished data from the manufacturer’s database. A Cochrane systematic review and meta-analysis identified reductions in HbA1c but no effect on morbidity or mortality.12 These controversies around acarbose are not mentioned in a more recent review by Hanefeld, who was also one of the STOP-NIDDM investigators.13
Other supporting laboratory evidence for mechanisms of possible cardiovascular benefit have come from studies in animals and humans. A randomised-controlled study in mice with placebo, sucrose and sucrose-acarbose showed exaggerated cardiac damage after ischaemia/reperfusion injury with repetitive postprandial hyperglycaemia that could be reduced with acarbose treatment.14 Reactive oxygen species and not altered neutrophil infiltration have been implicated in the enhanced myocardial injury. Postprandial hyperglycaemia has been shown to be associated with enhanced lipid peroxidation, platelet activation, and endothelial dysfunction in early type 2 diabetes, which could be attenuated with acarbose.15
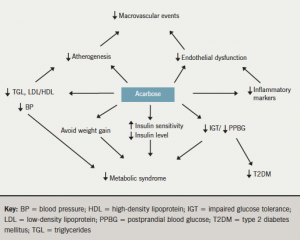
The possible cardiovascular benefits of acarbose are illustrated in figure 2.
The Effects of Acarbose Long-Term Therapy on Prevention of Cardiovascular Events in Abnormal Glucose Tolerance With Coronary Artery Disease (ALERT) study has examined the effects of acarbose in 300 Japanese patients with abnormal glucose tolerance who have undergone stenting for stable angina or acute coronary syndromes. The primary outcome is cardiovascular event-free survival time, with conversion from abnormal glucose tolerance to diabetes as a secondary outcome. The study lasted four years and the results are awaited. The Acarbose Cardiovascular Evaluation (ACE) trial is an ongoing four-year multi-centre, double-blind, randomised, parallel-group trial involving 7,500 patients looking at the effect of acarbose in reducing a primary cardiovascular composite end point in patients with impaired glucose tolerance and established cardiovascular disease. Again, conversion to diabetes is a secondary outcome.
Discussion
Acarbose acts by a unique mechanism of action compared with the rest of the antidiabetic drugs by its local action on the gastrointestinal system. Because of its poor systemic absorption it is a very safe drug and has no significant drug interactions. It has a very high percentage of gastrointestinal side effects, which are related to the carbohydrate in the meal. Its frequency of administration, cost and poor tolerability, as well as its comparatively weak potency, have made it the relatively least favoured choice. The drug has to be started at a very low dose and titrated upwards very slowly over several weeks to achieve the ideal tolerable dose, thereby limiting dropout rates. There is no failure of efficacy on long-term treatment unlike sulphonylureas.
It has significant efficacy in reducing postprandial hyperglycaemia and is indicated in situations where HbA1c is uncontrolled as a result of the abovementioned pathophysiology. It is one of the drugs approved for use in impaired glucose tolerance, and has achieved normoglycaemia, as well as halted the progression to type 2 diabetes mellitus. It is unsure if this is due to its masking of the diagnosis or true prevention of the disease.
Some studies have suggested possible beneficial cardiovascular outcomes, as well as improvement of multiple parameters including endothelial dysfunction, blood pressure, prevention of development of hypertension, reduction in cardiovascular events, and reduced fibrinogen, C-reactive protein and nuclear transcription factor. The possible cardiovascular benefits remain controversial, however, and we need the results of major long-term randomised-controlled trials to specifically address the cardiovascular benefits of acarbose.
Conflict of interest
GA and GAM: none declared. MF is an events
adjudicator for the ACE trial.
Key messages
- Acarbose can be used as monotherapy when other antidiabetic drugs have failed or are contraindicated
- Dose initiation and titration has to be very slow, over several weeks, to minimise side effects, improve compliance and optimise it to an ideal tolerable dose
- Acarbose use is not associated with treatment failure, weight gain or hypoglycaemia
- Possible cardiovascular benefits need to be proven in major randomised-controlled trials with primary cardiovascular outcomes
References
- Gentile S, Guarino G, Romano M et al. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol 2005;3:184–91.
- Riccardi G, Giacco R, Parillo M et al. Efficacy and safety of acarbose in the treatment of type 1 diabetes mellitus: a placebo-controlled, double-blind, multicentre study. Diabet Med 1999;16:228–32.
- Hoffman J, Spengler M. Efficacy of 24-week monotherapy with acarbose, glibenclamide, or placebo in NIDDM patients. The Essen Study. Diabetes Care 1994;17:561–6.
- Hoffman J, Spengler M. Efficacy of 24-week monotherapy with acarbose, metformin, or placebo in dietary treated NIDDM patients: The Essen-II Study. Am J Med 1997;103:483–90.
- Holman RR, Cull CA, Turner RC; on behalf of the UKPDS study group. A randomised double-blind trial of acarbose in type 2 diabetes shows improved glycaemic control over 3 years (UK Prospective Diabetes Study 44). Diabetes Care 1999;22:960–4.
- Buse J, Hart K, Minasi L. The PROTECT study: final results of a large multicentre postmarketing study in patients with type 2 diabetes. Precose Resolution of Optimal Titration to Enhance Current Therapies. Clin Ther 1998;20:257–69.
- Chiasson J-L, Josse RG, Gomis R et al.; for the STOP-NIDDM Trial Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–7.
- Chiasson J-L, Josse RG, Gomis R et al.; for the STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM trial. JAMA 2003;290:486–93.
- Hanefeld M, Chiasson JL, Koehler C et al. Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke 2004;35:1073–8.
- Kaiser T, Sawicki PT. Acarbose for prevention of diabetes, hypertension and cardiovascular events? A critical analysis of the STOP-NIDDM data. Diabetologia 2004;47:575–80.
- Hanefeld M, Cagatay M, Petrowitsch T et al. Acarbose reduces the risk of myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J 2004;25:10–16.
- Van de Laar FA, Lucassen PL, Akkermans RP et al. Alpha-glucosidase inhibitors for patients with type 2 diabetes. Results from a Cochrane systematic review and meta-analysis. Diabetes Care 2005;28:166–75.
- Hanefeld M. Cardiovascular benefits and safety profile of acarbose therapy in prediabetes and established type 2 diabetes. Cardiovasc Diabetol 2007;6:20.
- Frantz S, Calvillo L, Tillmanns J et al. Repetitive postprandial hyperglycaemia increases cardiac ischemia/reperfusion injury: prevention by the alpha-glucosidase inhibitor acarbose. FASEB J 2005;19:591–3.
- Shimabukuro M, Higa N, Chinen I et al. Effects of single administration of acarbose on postprandial glucose excursion and endothelial dysfunction in type 2 diabetic patients: a randomized crossover study. J Clin Endocrinol Metab 2006;91:837–42.

