Risk stratification is important in the assessment of cardiac patients enrolled in physical training programmes but is often based on inadequate information. Measuring blood B-type natriuretic peptide (BNP) level, a marker of left ventricular dysfunction, might improve risk assessment. In an observational study blood BNP levels were measured in 100 consecutive patients joining a cardiac rehabilitation programme following acute myocardial infarction. The results were compared with the clinical risk assessment – high, moderate or low. There was a significant correlation between risk category (high, moderate or low) and BNP level (r=0.41, p=0.001). A BNP level of 100 pg/L or more gave a sensitivity of 89% (95% confidence interval [CI] 0.69, 0.97) and a specificity of 61% (95% CI 0.57, 0.63) for predicting high-risk patients with a positive predictive value of 33% (95% CI 0.26, 0.36) and a negative predictive value of 96% (95% CI 0.89, 0.99). A BNP level of less than 100 pg/ml gave a sensitivity of 78% (95% CI 0.55, 0.91) and a specificity of 54% (95% CI 0.43, 0.64) for predicting low-risk patients with a positive predictive value of 27% (95% CI 0.17, 0.40) and a negative predictive value of 92% (95% CI 0.80, 0.97). In conclusion, BNP levels provide information that may improve the accuracy of risk assessment of cardiac rehabilitation patients particularly when other information is limited.
Introduction
Risk stratification is important in the assessment of cardiac patients enrolled in physical training programmes to ensure that these patients receive the appropriate levels of surveillance and exercise intensity. Risk levels, an estimate of the likelihood of future cardiac events, are indicated as low, moderate or high. Poor left ventricular (LV) function is the most important risk factor for death.1,2
The gold standard for assessing LV function is echocardiography but this is expensive and is often not available to cardiac rehabilitation co-ordinators. The additional information provided by plasma B-type natriuretic peptide (BNP) levels might be helpful. This has not been studied before.
BNP is excreted into the bloodstream by the ventricular wall when it is stretched and blood levels of BNP are, therefore, raised in patients with LV dysfunction and heart failure.3,4 Blood levels of BNP, or its precursor N-terminal proBNP, have been found to be the most significant predictors of prognosis in heart failure.5 A raised level of BNP after acute myocardial infarction is associated with increased risk of LV remodelling, heart failure and death.6-8 In the presence of a normal echocardiogram a raised BNP also has prognostic significance. A low level of BNP or N-terminal proBNP has been found to be highly predictive of normal LV function and good prognosis.7-10
The purpose of this study was to compare BNP level with risk assessment in a group of post-infarct patients joining a supervised exercise-based phase III cardiac rehabilitation programme.
Methods
One hundred consecutive post-infarct patients attending the cardiac rehabilitation programme after discharge from the North Hampshire Hospital coronary care unit were included. A post hocpower calculation indicated that with 100 patients and a sensitivity of predicting high-risk patients of 89%, the true sensitivity can be estimated to within a width of ±6%, with 95% confidence.
All the patients had suffered troponin positive myocardial infarction. Sixty-nine patients had had echocardiograms and 26 had had coronary angiography with ventriculography performed during their admission, but figures giving an estimate of ejection fraction were not available. However, the cardiologist assessing risk level was aware of the results of these investigations.
The patients joined the supervised exercise-based Phase III cardiac rehabilitation programme at three to four weeks after the attack. Blood was taken at their first attendance and BNP levels were measured from whole blood, using the Triage near-testing machine.11 (BNP levels are expressed as pg/ml and the cut-off point between normal and abnormal is taken as 100 pg/ml for both sexes.) The blood samples were refrigerated and tested within 24 hours. The BNP result was not known to any member of the cardiac rehabilitation clinical team.
Patients completed the Hospital Anxiety and Depression questionnaire12 to measure psychological state and the Dartmouth Primary Care Cooperative Information Project/World Organization of National Colleges, Academies, and Academic Associations of General Practice/Family Physicians (COOP-WONCA) questionnaire13 to measure quality of life. They then performed a submaximal treadmill exercise test to a modified Borg score (range 1 to 10) of between 6 and 8 and the predicted peak oxygen uptake was calculated (peak VO2). All exercise tests were supervised by the same cardiologist.
The cardiologist then assessed the risk from all the available clinical evidence using the criteria of established guidelines2 (which did not include the BNP level, of which he was unaware) and allocated each patient to a high-, moderate- or low-risk category.
Statistical analysis
Receiver operating characteristics (ROC) curves were used to assess the quality of the discriminatory power of BNP levels to predict high and low risk as assigned from clinical assessment. Sensitivity, specificity, and positive and negative predictive values were calculated at different cut-off points to ascertain the optimum sensitivity/specificity. All analyses were performed using SPSS version 1.4.0 (SPSS Inc., Chicago, USA).
Results
Of the 100 patients, 30 were women. Forty-three had been treated by percutaneous coronary intervention (PCI) shortly after the attack. The following were the characteristics that might influence BNP levels:
- The age range was 41 to 84 years, mean 63 years. (BNP levels rise with increasing age.14,15)
- Body mass index ranged from 19.0 to 51.7 kg/m2, mean 28.1 kg/m2. Twenty-two patients had body mass indices between 30.0 and 34.9 kg/m2, three between 35.0 and 39.9 kg/m2 and three greater than 40.0 kg/m2. (BNP levels fall with rising body mass index.16)
- Estimated glomerular filtration rates (eGFR) were performed for all patients during their admission. Three had levels below 30 ml/minute, indicating severe renal disease. (BNP rises with deteriorating renal function.14,15)
- No patients had uncontrolled hypertension or severe lung disease, though one had diagnosed chronic obstructive pulmonary disease and seven had asthma. (BNP is excreted by the stretched right ventricle as well as with the left.)
- Cardiac troponin taken shortly after the attack ranged from 0.16 to >50 µg/L, mean 14.2 µg/L. (BNP levels rise with increasing cardiac damage.7)
- Ninety patients were receiving either angiotensin-converting enzyme (ACE) inhibitors or angiotensin blocking agents, which may reduce BNP levels.
Table 1 summarises the relationship between risk category attributed to the patients and BNP level, age, peak oxygen uptake (peak VO2) and infarct-related troponin level.

There were 18 patients in the low-risk group, 64 in the moderate-risk group and 18 in the high-risk group. There is a clear trend for BNP levels to increase as we move from the low- to the high-risk category. Age tends to increase with risk category as does troponin, whereas peak VO2 decreases as risk increases.

Figure 1 is the ROC curve for predicting high risk from BNP levels. Table 2 shows the diagnostic characteristics of BNP levels at different cut-off points. The optimum level of BNP to predict high risk was 100 pg/ml or more which gave a sensitivity of 89% (95% confidence interval [CI] 0.69, 0.97) and a specificity of 61% (95% CI 0.57, 0.63) with a positive predictive value for being in the high-risk category of 33% (95% CI 0.26, 0.36) and a negative predictive value of 96% (95% CI 0.89, 0.99).
Figure 2 is the ROC curve for predicting low risk from BNP levels. Using a BNP level of <100 pg/ml to predict low risk gave a sensitivity of 78% (95% CI 0.55, 0.91) and a specificity of 54% (95% CI 0.43, 0.64) with a positive predictive value of 27% (95% CI 0.17, 0.40) and a negative predictive value of 92% (95% CI 0.80, 0.97).
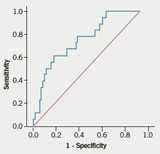
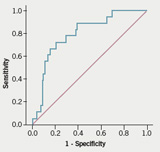
Two patients in the high-risk category had BNP levels below 100 pg/ml. One was included in this category because she had suffered cardiogenic shock in the coronary care unit, with a very high BNP level in hospital of 920 pg/ml (this figure was not known to the clinical team) and echocardiographic evidence of ‘severe left ventricular damage’ – but had subsequently been found to have normal coronary arteries and no evidence of permanent LV damage. The second had been included in this category because he had suffered an anterior Q-wave myocardial infarction with a low exercise tolerance when he first attended cardiac rehabilitation. His echocardiogram indicated ‘mildly impaired left ventricular systolic function’.
Four patients in the low-risk category had BNP levels higher than 100 pg/ml and none had a BNP level higher than 270 pg/ml. All had been able to perform more than six minutes of the Bruce protocol on the treadmill but one had had an extensive right ventricular infarct, another had suffered a previous infarct and a third was over the age of 80 years.
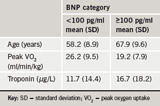
Table 3 shows summary statistics for the relationship between BNP levels and risk category – age, fitness level and infarct related troponin level. Patients with BNP ≥100 pg/ml were, on average older, had lower levels of peak VO2 and higher post-infarct troponin levels.
No patients were re-admitted during the course of the study and 10 patients suffered problems during the exercise programme including breathlessness, chest pain and hypotension. All had been included in the moderate-risk category but four had BNP levels above 300 pg/ml.
Discussion
This study demonstrates a relationship between BNP levels and risk assessment in patients joining a cardiac rehabilitation programme following acute myocardial infarction. Patients with a BNP level below 100 pg/ml are unlikely to be at high risk and those with a BNP level of 300 pg/ml or more are almost certainly at medium or high risk. In most cases the increased risk is associated with greater LV damage. In this group of patients the BNP level would, if known to the clinicians involved, have resulted in reclassification of one apparently high-risk and three apparently low-risk patients.
The optimum cut-off level for BNP of ≥100 pg/ml which we found for detecting the likely correlations between BNP and risk is similar to the findings from previous work. Studies of BNP levels in patients with heart failure have suggested that levels below 100 pg/ml made the diagnosis of heart failure unlikely, whereas levels above 500 pg/ml make it very likely.17 In acute dyspnoea a cut-off level of 100 pg/ml gave the best sensitivity and specificity for distinguishing between heart failure and other causes for breathlessness.18 In stable coronary heart disease figures of between 52 and 100 pg/ml have been found to give the best cut-off values for predicting reduced LV ejection fraction or subsequent mortality.4,19,20 Following acute myocardial infarction, the optimal cut-off levels for predicting heart failure or death have been found to lie between 52 and 180 pg/ml.4,21,22 One study of remodelling in a group of acute myocardial infarction patients undergoing cardiac rehabilitation found that LV remodelling occurred exclusively in those with a BNP above 150 pg/ml at the start of the programme.23
In this study, the risk assessment was made by a cardiologist who had access to much more information than is available to most cardiac rehabilitation co-ordinators in the UK, i.e. physical findings and treadmill exercise test performance and, in most cases, the results of previous echocardiography or ventriculography. Even so the BNP level, had it been known, would have changed the risk assignation in one high-risk and three low-risk patients. The identification of risk category of cardiac rehabilitation patients is important. The exercise training of cardiac patients should be tailored to the individual patient and depends upon the needs, ability and level of risk. Those at high risk need more gradual increase in exercise duration and intensity and a longer period of supervised training before they are safe to be discharged to exercise without supervision. On the other hand low-risk patients need very little monitoring and can safely be discharged from formal supervised Phase III cardiac rehabilitation into unsupervised Phase IV exercise after a short period of supervision. Where facilities are at a premium, low-risk patients may be sent straight into Phase IV while the limited resources of the Phase III cardiac rehabilitation programme are concentrated on higher-risk patients. Most cardiac rehabilitation centres in the UK tend to take on relatively low-risk patients with very low long-term mortality yet do not have the capacity to treat more than a fraction of those who might benefit.24 BNP level should not be used on its own as an indicator of risk since other factors provide additive information.25 However, routine measurement of BNP levels might allow co‑ordinators to identify with confidence low-risk patients so that they could free up space on the programme to take on a greater proportion of eligible patients, more of whom would be the high-risk patients who have the most to gain from cardiac rehabilitation.
The usefulness of BNP as an indicator of risk may be limited by influences other than LV damage. BNP levels are higher in women than men.15 N-terminal proBNP level but not BNP levels rise with age in subjects with normal hearts. The positive correlation between age and BNP in this study was probably due to worsening of LV function with increasing age.
There are several limitations of this study. The sample size is small, with only 18 patients falling into each of the high- and low-risk categories. With the limited number of patients and the limited follow-up there were insufficient adverse events to validate the risk assessments as predictors of death or future events. We have, therefore used the clinical risk assessment, the best available predictor of prognosis, as the gold standard against which to compare the predictive power of BNP level. Our assessment of the utility of BNP level to predict risk may be confounded by age and by exercise capacity and post-infarct troponin level. The small sample size prevented the use of modelling techniques to try to account for these potential confounders. We have used BNP rather than N-terminal proBNP levels, which may be more readily available in centres where measurements are made by the pathology laboratories. However, evidence suggests that the two give very similar risk prediction though the blood levels of N-terminal proBNP are approximately three times those of BNP26.
Conflicts of interest
None declared.
Ethical approval
Ethical approval for this study was granted by North & Mid Hampshire Local Research Ethics Committee on 17 April 2003.
Acknowledgement
Dr Hugh Bethell is in receipt of National Health Service Research & Development funding.
Key messages
- Risk stratification is important in the assessment of cardiac rehabilitation patients
- The information needed for accurate risk stratification is not always available to cardiac rehabilitation co‑ordinators
- B-type natriuretic peptide (BNP) blood levels are closely related to risk assessment levels
- BNP levels may help to improve the accuracy of risk stratification in cardiac rehabilitation patients
References
- Coats A, McGhee H, Stokes H, Thompson D. British Association for Cardiac Rehabilitation Guidelines. Oxford: Blackwell Science, 1995.
- American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for cardiac rehabilitation and secondary prevention programs. 4th Edition. Champain, Ill: Human Kinetics, 2004.
- Magga J, Vuolteenaho O, Tokola H et al. B-type natriuretic peptide: a myocyte-specific marker for characterizing load-induced alterations in cardiac gene expression. Ann Med1998:30(suppl 1):39–45.
- Choy AM, Darbar D, Lang CC et al. Detection of left ventricular dysfunction after acute myocardial infarction: comparison of clinical, echocardiographic and neurohormonal methods. Br Heart J 1994;72:16–22.
- Doust JA, Pietrazak E, Dobson A, Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ 2005;330:625.
- Nagaya N, Goto Y, Nishikimi T et al. Sustained elevation of plasma brain natriuretic peptide levels associated with progressive ventricular remodelling after acute myocardial infarction. Clin Sci 1999;96:129–36.
- Richards AM, Nicholls MG, Yandle TG et al. The Christchurch Cardioendocrine Research Group. Neuroendocrine prediction of left ventricular function and heart failure after acute myocardial infarction. Heart 1999;81:114–20.
- Crilley JG, Farrer M. Left ventricular remodelling and brain natriuretic peptide after first myocardial infarction. Heart 2001;86:638–42.
- Smith H, Pickering RM, Struthers A, Simpson I, Mant D. Biochemical diagnosis of ventricular dysfunction in elderly patients in general practice: observational study. BMJ 2000;320:906–08.
- Davidson NC, Naas AA, Hanson JK, Kennedy NSJ, Coutie WJ, Struthers AD. Comparison of atrial natriuretic peptide, B-type natriuretic peptide and N-terminal proatrial natriuretic peptide as indicators of left ventricular systolic dysfunction. Am J Cardiol 1996;77:828–31.
- Cheng V, Kazanagra R, Garcia A et al. A rapid bedside test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol 2001;37:386–91.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361–70.
- Nelson E, Wasson J, Kirk J et al. Assessment of function in routine clinical practice: description of the COOP chart method and preliminary findings. J Chron Dis 1987;40(suppl 1):55S–63S.
- Omland T, Aakvaag A, Bonarjee VVS et al. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation 1996;93:1963–9.
- Loke I, Squire IB, Davies JE, Ng LL. Reference ranges for natriuretic peptides for diagnostic use are dependent on age, gender and heart rate. Eur J Heart Fail 2003;5:599–606.
- Wang TJ, Larson MG, Levy D et al. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004;109:594–600.
- Mueller C, Scholer A, Laule-Kilian K et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med 2004;350:647–54.
- McCullough PA, Nowak RM, McCord J et al. B-type natriuretic peptide and clinical judgement in emergency diagnosis of heart failure. Analysis from breathing not properly (BNP) multinational study. Circulation 2002;106:416–22.
- Omland T, Richards AM, Wergeland R, Vik-Mo H. B-type natriuretic peptide and long-term survival in patients with stable coronary artery disease. Am J Cardiol 2005;95:24–8.
- Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. Is B-type natriuretic peptide a useful screening test for systolic or diastolic dysfunction in patients with coronary disease? Data from the Heart and Soul Study. Am J Med 2004;116:509–16.
- Nicholls MG, Obineche EN, Frampton CM, Richards AM. Plasma cardiac natriuretic peptide levels in screening for cardiac disease. Am J Med 2004;116:561–3.
- Morita E, Yasue H, Yoshimura M et al. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation 1993;88:82–91.
- Tagaki S, Sakuragi S, Baba T et al. Predictors of left ventricular remodelling in patients with acute myocardial infarction participating in cardiac rehabilitation. Circ J 2004;68:214–19.
- Bethell HJN, Turner SC, Evans JA. Cardiac rehabilitation in the UK 2000 – can the National Service Framework milestones be attained? Br J Cardiol 2004;11:162–8.
- De Groote P, Dagorn J, Soudan B, Lamblin N, McFadden E, Bauters C. B-type natriuretic peptide and peak exercise oxygen consumption provide independent information for risk stratification in patients with stable congestive heart failure. J Am Coll Cardiol 2004;43:1584–9.
- Mueller T, Gegenhuber A, Poelz W, Haltmeyer M. Diagnostic accuracy of B type natriuretic peptide and amino terminal proBNP in the emergency diagnosis of heart failure. Heart2005;91:606–12.
