Correction of thyroid hormone levels using thyroxine can have important cardiac implications. We report a case of myocardial infarction following rapid up-titration of thyroxine.
Case history
A 45-year-old woman was admitted with worsening confusion, lethargy, unsteadiness on her feet and hallucinations. She had a past medical history of depression and agoraphobia and had become progressively more withdrawn and insular over a 14-year period. She had not seen her general practitioner for several years and was housebound, to the extent that her bed had to be moved downstairs. Her condition gradually deteriorated and she required admission to hospital.
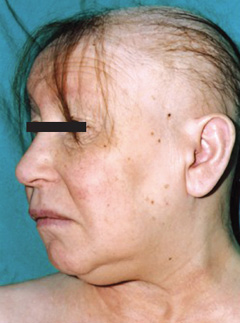
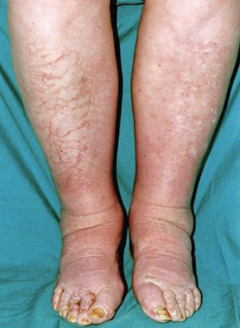
On admission, she was obese with marked alopecia, coarse dry skin, ‘lemon and peach’ skin complexion, had peri-orbital puffiness, and madarosis (figure 1), as well as non-pitting oedema of lower limbs (figure 2). Her vital status was stable with a blood pressure of 145/90 mmHg, pulse 55 per minute sinus rhythm but she was hypothermic with a temperature of 33.9°C. Neurological findings were consistent with reduced power in all four limbs (grade 3/5), and absent reflexes globally and she scored 5/10 on the abbreviated mini-mental test score. Investigations including a full blood count, chemistry and electrocardiogram (ECG) were all within normal limits.
A provisional diagnosis of hypothyroidism was made, which was confirmed by biochemical testing. The thyroid stimulating hormone (TSH) was >110 mU/L (normal 0.5–5 mU/L), and the free thyroxine (T4) was 2.5 mU/L and thyroid autoantibody testing was negative. The patient was started on levothyroxine 50 µg once daily and three days later this was titrated up to 100 µg once daily and then finally to 125 µg once daily a week later. The patient improved symptomatically, started to lose weight, had improved appetite and became more alert and oriented. She was discharged home after a four-week in-patient stay.
The patient was re-admitted to the Accident and Emergency department three days later, having experienced ischaemic chest pain for some eight hours prior to admission. Her presenting ECG showed ST elevation of 1 mm in leads II, III, AVF and ST elevation of 2 mm in V6, consistent with a diagnosis of inferior myocardial infarction. The patient’s pain was controlled with morphine and she did not receive fibrinolytic therapy as subsequent ECGs done an hour after presentation had shown resolution of the initial ST elevation. She was treated conservatively with aspirin 75 mg, clopidogrel 300 mg as a loading dose followed by 75 mg once daily, atorvastatin 80 mg once daily, bisoprolol 2.5 mg once daily, ramipril 5 mg twice daily and subcutaneous low molecular weight heparin. Her only identifiable risk factor was smoking. The myocardial infarction was attributed to the recent and rapid increase in thyroxine replacement therapy in a profoundly myxodematous patient. In addition to usual post-myocardial infarction care, the dose of thyroxine was reduced to 25 µg once daily, with a view to increase the dose by 25 µg every two months to achieve euthyroid status. An in-patient echocardiogram revealed infero-posterior wall hypokinesia but with reasonably preserved overall left ventricular function. Coronary angiography revealed moderate two vessel disease, which was managed medically.
Discussion
We present a case of iatrogenic myocardial infarction resulting from rapid correction of a chronic metabolic abnormality. Thyroxine has a prolonged half-life (seven days), and hence steady state concentrations of the hormone occur four to six weeks after any dosing change. Therefore, biochemical testing and further dose changes should not be performed at intervals of less than four to six weeks. Furthermore, thyroxine increases oxygen consumption and most peripheral tissues contribute to this response, including the heart, skeletal muscle, liver and kidney. The heart is particularly sensitive, and 30–40% of the thyroid hormone-dependent increase in oxygen consumption is due to stimulation of cardiac contractility.1-3
In subjects with long-standing hypothyroidism, the goal of thyroxine replacement is to achieve a TSH level in the normal range over a period of three to six months. Rapid, aggressive correction, can lead to a number of cardiac abnormalities including arrhythmias and myocardial ischaemia.4 In particular, individuals over the age of 60, who may have occult coronary artery disease, should be carefully assessed and receive replacement therapy at lower daily doses over an extended period of time in some cases. Such an approach may avoid exacerbation of underlying or undiagnosed cardiac disease.5,6
This case highlights this important point and should remind clinicians that, when presented with long-standing hypothyroidism, thyroxine replacement should be optimised over months rather than days.
Conflict of interest
None declared.
Key messages
- In patients with long-standing hypothyroidism, particularly over the age of 60 years, careful consideration should be given to the presence of occult coronary artery disease
- In patients with long-standing hypothyroidism, optimisation of thyroxine replacement should be a slow gradual process over months rather than weeks
- Patients recently commenced on thyroxine treatment for long-standing hypothyroidism should be advised to seek urgent medical advice if they experience chest pain after treatment is commenced
References
- Goodman L, Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, Ninth Edition. New York: McGraw-Hill, 1996.
- Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res 2004;59:31–50.
- Klein I, Ojamaa K. Mechanisms of disease: thyroid hormone and the cardiovascular system. N Engl J Med 2001;344:501–09.
- Kohno A, Hara Y. Severe myocardial ischemia following hormone replacement in two cases of hypothyroidism with normal coronary arteriogram. Endocrin J 2001;48:565–73.
- Auer J, Berent R, Weber T, Lassing E, Eber B. Thyroid function is associated with presence and severity of atherosclerosis. Clin Cardiology 2003;26:569–73.
- Hiasi Y, Aihara T, Bando M, Nakai Y. Acute myocardial infarction due to coronary spasm associated with L-thyroxine therapy. Clin Cardiology 1989;12:161–3.
