Co-administered niacin and statin may offer additional lipid management; however, niacin is underutilised due to flushing, mediated primarily by prostaglandin D2 (PGD2). A combination tablet containing 1 g extended-release niacin and 20 mg laropiprant (ERN/LRPT), a PGD2-receptor (DP1) antagonist, offers improved tolerability. To assess the efficacy and safety of ERN/LRPT + simvastatin versus ERN/LRPT and simvastatin alone in dyslipidaemic patients, in this 12-week study, 1,398 patients were randomised equally to ERN/LRPT 1 g/20 mg, simvastatin (10, 20 or 40 mg), or ERN/LRPT 1 g/20 mg + simvastatin (10, 20 or 40 mg) once-daily for four weeks. At week five, doses were doubled in all groups except simvastatin 40 mg (unchanged) and ERN/LRPT 1 g/20 mg + simvastatin 40 mg (switched to ERN/LRPT 2 g/40 mg + simvastatin 40 mg).
ERN/LRPT + simvastatin (pooled across simvastatin doses) significantly improved key lipid parameters versus ERN/LRPT and pooled simvastatin: mean percentage changes from baseline to week 12 for low-density lipoprotein cholesterol were –47.9%, –17.0% and –37.0%, respectively, and for high-density lipoprotein cholesterol were 27.5%, 23.4% and 6.0%, respectively. ERN/LRPT + simvastatin was generally well tolerated, with a low incidence of serious treatment-related adverse experiences (0.2%, 0.5% and 0.2% for ERN/LRPT + simvastatin, ERN/LRPT and simvastatin, respectively).
In conclusion, ERN/LRPT + simvastatin significantly improved the lipid profile compared with ERN/LRPT and simvastatin alone and was generally well tolerated in dyslipidaemic patients.
Introduction
Large intervention studies suggest that while lowering low-density lipoprotein cholesterol (LDL-C) is beneficial, it is insufficient to prevent the majority of coronary heart disease (CHD) events. Niacin improves the overall lipid profile, LDL-C and triglycerides (TG) and is the most effective agent for raising high-density lipoprotein cholesterol (HDL-C) levels.1 Co-administration of niacin with a statin offers the potential for additional lipid management, but the use of niacin has been limited due to associated flushing, mediated primarily by prostaglandin D2 (PGD2).2 Flushing of the face and trunk occurs in nearly all patients taking niacin.3-5 The PGD2-mediated pathway is independent of the pathway underlying niacin’s beneficial lipid-modifying effects,6 enabling the development of an agent that inhibits PGD2-mediated flushing without interfering with the beneficial lipid-modifying effects of niacin. Such an agent may facilitate more widespread use of niacin therapy to reduce cardiovascular risk in patients with dyslipidaemia.
Laropiprant (LRPT; Merck & Co., Inc., Whitehouse Station, NJ, USA) is a potent, once-daily, highly selective PGD2-receptor (DP1) antagonist.7 A combination tablet containing 1 g of extended-release niacin and 20 mg of laropiprant (ERN/LRPT) offers improved tolerability, supporting a simplified 1–2 g dosing paradigm and improved adherence.8,9 In a randomised, placebo-controlled clinical trial, ERN/LRPT, administered alone or co-administered with a statin to 1,600 patients with primary hypercholesterolaemia or mixed dyslipidaemia, produced significant, durable improvements in multiple lipid and lipoprotein parameters and offered improved tolerability over ERN alone.9 The primary purpose of the present phase III factorial study was to assess the efficacy and safety of ERN/LRPT co-administered with simvastatin (ERN/LRPT+SIMVA) in patients with primary hypercholesterolaemia or mixed hyperlipidaemia.
Patients and methods
Patient selection criteria
This study enrolled men and women ages 18 to 85 years with primary hypercholesterolaemia or mixed hyperlipidaemia (0–1 risk factor according to National Cholesterol Education Program Adult Treatment Panel III [NCEP ATP III] guidelines1 with an LDL-C from 130 to 190 mg/dL [3.37 mmol/L to 4.92 mmol/L] or multiple NCEP ATP III risk factors with an LDL-C from 130 to 160 mg/dL [3.37 mmol/L to 4.14 mmol/L] and TG ≤350 mg/dL [3.96 mmol/L] after washout from lipid-modifying therapies). High-risk patients (CHD/CHD risk equivalent, including diabetes, per NCEP ATP III guidelines1) were intended to be excluded by protocol, as washout of statin was required. Patients were also excluded if they had the following laboratory values at visit one: creatinine >2.0 mg/dL (176.8 μmol/L), alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) >1.5x upper limit of normal (ULN), creatine kinase (CK) >2 x ULN, and thyroid stimulating hormone (TSH) outside the central laboratory’s normal reference range. Concomitant drugs excluded at study entry included: niacin ≥50 mg/day; lipid-modifying therapy initiated within six weeks of visit one; fibrates; cyclical hormonal contraceptives or intermittent use of hormone replacement therapies; systemic corticosteroids; or high-dose antioxidant vitamins.
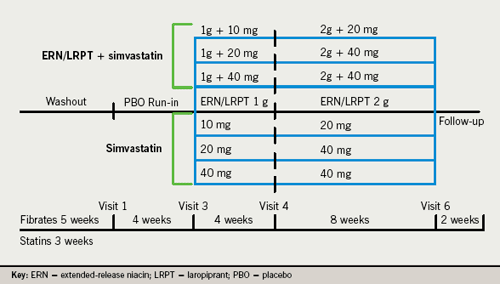
Study design
This was a worldwide, multi-centre, randomised, double-blind, factorial design, 12-week parallel-group study conducted at 108 sites in 15 countries. Following a six- to eight-week washout period of lipid-modifying therapies (if needed) and a concurrent four-week placebo run-in, patients were equally randomised to one of seven treatment arms: ERN/LRPT 1 g/20 mg, SIMVA (10, 20 or 40 mg), or ERN/LRPT 1 g/20 mg+SIMVA (10, 20 or 40 mg) once-daily for four weeks. At week five, all doses were doubled (two tablets) except SIMVA 40 mg (unchanged) and ERN/LRPT 1 g/20 mg+SIMVA 40 mg (switched to ERN/LRPT 2 g/40 mg+SIMVA 40 mg) for eight weeks (figure 1). Patients were instructed to take study therapy with their evening meal.
The study protocol was reviewed and approved by the appropriate ethics committees/institutional review boards. All patients provided written informed consent to participate in this study. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice standards.
Efficacy assessments
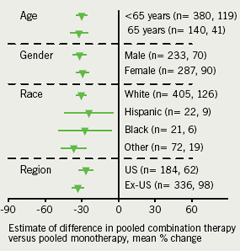
The primary efficacy end point was efficacy of co-administered ERN/LRPT 2 g+SIMVA pooled across 20 and 40 mg doses (pooled) compared with ERN/LRPT 2 g on percentage change from baseline in LDL-C levels at week 12. Key secondary efficacy end points included efficacy of co-administered ERN/LRPT 2 g+SIMVA (pooled) compared with SIMVA (pooled) on LDL-C, HDL-C, TG, ratio of LDL-C:HDL-C, non-HDL-C, apolipoprotein (Apo) B and Apo A-I, and efficacy of co-administered ERN/LRPT 2 g+SIMVA (pooled) compared with ERN/LRPT 2 g on HDL-C, TG, ratio of LDL-C:HDL-C, non-HDL-C, Apo B and Apo A-I. Exploratory end points included percentage change from baseline in total cholesterol (TC), ratio of TC:HDL-C, lipoprotein (a) (Lp(a)), and C-reactive protein (CRP). The treatment effects on LDL-C at study end point were examined across pre-specified subgroups defined by age (< or ≥65 years), gender, race (White, Hispanic, Black or other), and region (US or ex-US).
Safety and tolerability assessments
Safety and tolerability assessments included adverse events (AEs), physical examinations, laboratory tests, electrocardiograms (ECGs) and vital signs. Laboratory evaluations included serum ALT, AST, CK, fasting serum glucose (FSG), and creatinine and other general surveillance laboratory tests. For patients without diabetes mellitus at baseline, the percentage of patients with new-onset diabetes mellitus, defined as experiencing a diabetes-related AE or addition of an antihyperglycaemic medication, was tabulated.
The following laboratory abnormalities were pre-specified as conditions for discontinuation: consecutive ALT or AST elevations ≥3 x ULN; consecutive CK elevations ≥5 x and <10 x ULN with muscle symptoms, consecutive CK elevations ≥10 x ULN with or without muscle symptoms, or single CK elevations ≥20 x ULN with or without muscle symptoms; TG levels >600 mg/dL (6.6 mmol/L) on repeat measure; and positive pregnancy test. Pre-specified discontinuation was also defined for patients who experienced hypersensitivity or severe intolerance to study therapy or who required continuous treatment with systemic corticosteroids.
Statistical methods
Percentage change from baseline in LDL-C at week 12 with ERN/LRPT 2 g+SIMVA (pooled) versus ERN/LRPT 2 g was assessed using an analysis of co-variance (ANCOVA) model, with terms for treatment, country, gender, and baseline LDL-C as co-variates. Percentage changes from baseline in other lipid end points (LDL-C, HDL-C, non-HDL-C, Apo B, Apo A-I, TC, ratios of LDL-C:HDL-C and TC:HDL-C) were analysed using a similar ANCOVA model, substituting the relevant baseline measurement as the co-variate. The comparisons were performed using appropriate contrasts from the ANCOVA model.
Percentage changes from baseline in TG, Lp(a) and CRP were analysed using non-parametric methods based on medians. In the pooled comparisons of ERN/LRPT+SIMVA vs. SIMVA or ERN/LRPT alone, the pair of compared treatments was analysed separately using the ANCOVA model applied to the Tukey’s normalised ranks of the percentage change from baseline, while the Tukey’s normalised ranks of baseline level was used as a co-variate. Pair-wise comparisons were performed using the respective contrast from the ANCOVA model. Between-treatment group differences in medians were assessed using Hodges-Lehman estimates with the corresponding distribution-free 95% confidence interval (CI) based on Wilcoxon’s rank-sum test applied to the pair of compared treatments. Individual dose comparisons (unpooled) were analysed similarly using the pair of compared treatment groups.
Safety and tolerability were assessed by a statistical and clinical review of all safety parameters. Statistical tests were performed and the 95% CI and p-values were displayed on the pre-specified safety parameters of interest. For all other clinical and laboratory AEs, events were listed and summarised by frequency of occurrence, and the counts and percentages were tabulated by treatment group. The between-group pair-wise comparisons were performed using Fisher’s exact test. Ninety-five per cent CIs of between-treatment differences in percentages were derived using Wilson’s score method.
Results
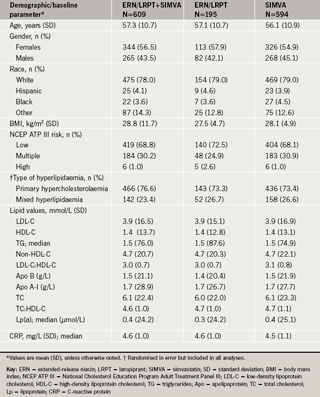
Demographics, baseline characteristics and patient accounting
The demographics and baseline characteristics were generally similar across the treatment groups (table 1). Overall, 3,302 patients were screened and, of these, 1,398 patients were randomised and 1,135 (81.2%) patients completed the study.
Lipid efficacy
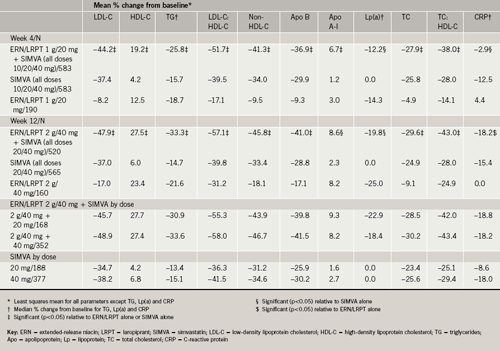
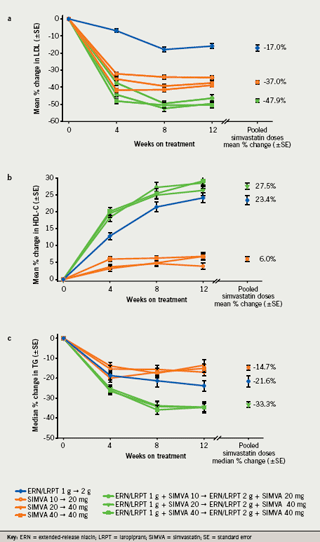
ERN/LRPT 2 g+SIMVA (pooled) produced significantly greater mean percentage reductions from baseline to week 12 in LDL-C compared with both ERN/LRPT 2 g and SIMVA (pooled) monotherapy groups (table 2; figure 2a). Individual dose comparisons demonstrated that co-administered ERN/LRPT+SIMVA produced significantly greater reductions in LDL-C versus both agents alone (p<0.001 for each individual pair-wise comparison). All of the respective comparisons (pooled and individual doses) were statistically significant at week four in favour of ERN/LRPT 1 g+SIMVA (table 2).
The LDL-C-lowering efficacy of co-administered ERN/LRPT 2 g+SIMVA (pooled) versus ERN/LRPT 2 g and SIMVA (pooled) at week 12 was generally consistent across subgroups defined by age (<65 or ≥65 years), gender, race (Caucasian, Hispanic, Black or other), and region (US or ex-US) (figure 3). Patients who had higher than median baseline TG levels experienced less of a percentage decline in LDL-C compared with patients with levels below the median in the treatment groups receiving ERN/LRPT; however, the absolute magnitudes of decline were similar in both subgroups, approximately 18 mg/dL (0.5 mmol/L).
Co-administered ERN/LRPT 2 g+SIMVA (pooled) produced significant improvements in the following lipid variables compared with ERN/LRPT 2 g and SIMVA monotherapy (pooled) at week 12: HDL-C (table 2; figure 2b), TG (table 2; figure 2c), LDL-C:HDL-C, non-HDL-C, Apo B, Apo A-I, TC, and TC:HDL-C (p<0.001 for each pair-wise between-group comparison; table 2). All of the respective comparisons (pooled and individual doses) were statistically significant at week four in favour of ERN/LRPT 1 g+SIMVA (table 2).
ERN/LRPT, administered either alone or with SIMVA, produced similar reductions in Lp(a), whereas SIMVA alone had no effect on Lp(a) (table 2). ERN/LRPT+SIMVA (pooled) did not produce additional reductions in CRP beyond SIMVA alone and ERN/LRPT appeared to have no effect on CRP levels (table 2).
Safety
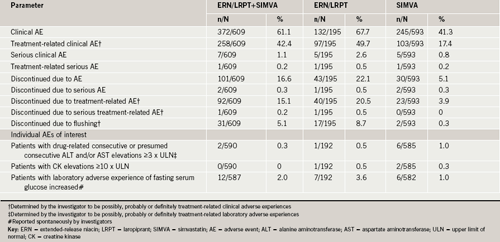
Co-administration of ERN/LRPT 2 g+SIMVA was generally well tolerated, having an overall AE profile similar to that of ERN/LRPT alone (table 3). The discontinuation rate was low in all seven treatment groups and primarily driven by flushing-related symptoms and gastrointestinal disorders in the ERN/LRPT treatment groups.
The percentages of patients with consecutive ≥3 x ULN elevations (including presumed consecutive elevations) in ALT/AST were low and similar across treatment groups (table 3). There was only one discontinuation in the study due to ALT and/or AST elevation, which occurred in the SIMVA monotherapy treatment arm. There was one report of hepatitis occurring in the ERN/LRPT+SIMVA treatment arm, which was ascribed to alcohol abuse and considered by the investigator to be definitely not related to study therapy.
There were no AEs of myopathy (CK ≥10 x ULN with muscle symptoms and considered drug-related by the investigator). Elevations in CK ≥10 x ULN were low and similar across treatment groups (table 3). One patient in the ERN/LRPT group had an elevation with muscle symptoms attributed to exercise and not considered to be treatment related. Two patients in the SIMVA (pooled) group discontinued due to elevated CK.
Adverse experiences related to glycaemic control (e.g. diabetes mellitus, impaired fasting glucose, insulin resistance) were reported by two (0.3%) patients in the ERN/LRPT+SIMVA (pooled) group, one (0.5%) patient in the ERN/LRPT group, and two (0.3%) patients in the SIMVA (pooled) group. There were no serious events or discontinuations related to glycaemic control AEs. Median increases in FSG were 4 mg/dL (0.22 mmol/L) in each individual ERN/LRPT+SIMVA group and the ERN/LRPT 2 g group, and ranged from 1 to 2 mg/dL (0.05 mmol/L to 0.11 mmol/L) in the SIMVA groups (table 3). Of the 1,397 patients without diabetes at baseline, four patients met the criteria for new-onset diabetes, two (1.0%) in the ERN/LRPT+SIMVA (pooled) group, one (0.5%) in the ERN/LRPT group, and one (0.5%) in the SIMVA (pooled) group.
Serum uric acid levels increased slightly from baseline to week 12 in the ERN/LRPT (0.5 mg/dL [0.02 mmol/L]) and ERN/LRPT+SIMVA (0.4 mg/dL [0.02 mmol/L]) treatment groups compared with SIMVA monotherapy (–0.1 mg/dL [~0.0 mmol/L]). Two cases of gout were reported as AEs in the ERN/LRPT+SIMVA treatment groups.
Discussion
Statins provide excellent LDL-C lowering effects; however, many patients fail to attain their LDL-C goals and frequently require combination therapy to reach increasingly aggressive targets. In addition, treatment of mixed hyperlipidaemia, characterised by elevated LDL-C and TG and low HDL-C levels, often with accompanying obesity, diabetes and metabolic syndrome, remains difficult to manage effectively with statin monotherapy. While statins effectively lower LDL-C and reduce cardiovascular risk by approximately 30%, they produce only modest effects on TG and HDL-C. Niacin produces beneficial effects on LDL-C, HDL-C, TG and other lipid and lipoprotein parameters and has been shown in clinical trials to reduce cardiovascular events and atherosclerosis progression.1,10-14 The combination of statins and niacin may produce additional and complementary lipid/lipoprotein effects, further reduce cardiovascular risk relative to either monotherapy, and provide an effective treatment option for patients with primary and mixed hyperlipidaemia.
In the present study, co-administration of ERN/LRPT 2 g+SIMVA (pooled) produced greater reductions in LDL-C than either monotherapy. Similar to the findings in the pooled treatment groups, all individual dose comparisons of ERN/LRPT 2 g+SIMVA produced significantly greater LDL-C reductions than the respective monotherapy doses. Despite that, the full effect of the 1 g dose may not have reached a plateau at four weeks, incremental LDL-C lowering was observed when the dose was advanced from 1 g to 2 g at week four. Based on previously published data, ERN/LRPT produced the expected effects on LDL-C observed with niacin as monotherapy and when co-administered with a statin.15
The LDL-C-lowering efficacy of the co-administration compared with ERN/LRPT alone was consistent across pre-defined subgroups based on gender, age, race and region. Patients in the ERN/LRPT groups with baseline TG levels above the median experienced a lower percentage decline in LDL-C relative to patients with levels below the median. Nonetheless, the absolute magnitudes of decline were similar. This finding is consistent with the known effects of baseline TG levels in patients receiving niacin therapy. Since patients were required to have TG ≤350 mg/dL (3.96 mmol/L) at baseline, the observed effects on LDL-C may not be generalisable to patients with severe hypertriglyceridaemia.
Apo B, the principle apolipoprotein of LDL-C, demonstrated an almost identical pattern of results as LDL-C, with greater reductions observed in the ERN/LRPT 2 g+SIMVA (pooled) compared with the ERN/LRPT and pooled SIMVA monotherapy groups. Since each LDL particle carries one molecule of Apo B, this parameter represents a measure of LDL particle number. Interestingly, ERN/LRPT produced commensurate reductions in LDL-C and Apo B, with greater reductions in TG, while SIMVA produced larger reductions in LDL-C compared with Apo B and TGs. The clinical meaning of this finding is unclear, but suggests that niacin may produce a greater relative effect on reducing particles that contain TGs, such as very low-density lipoprotein (VLDL) and intermediate-density lipoprotein (IDL), but which contain less cholesterol than LDL particles. Similar to the changes observed in LDL-C and Apo B, ERN/LRPT 2 g+SIMVA (pooled) produced a significantly greater reduction in non-HDL-C, a marker for atherogenic Apo B-containing lipoproteins, than SIMVA (pooled) and ERN/LRPT 2 g alone. Similar results were observed for individual SIMVA dose comparisons, with or without ERN/LRPT.
ERN/LRPT 2 g+SIMVA (pooled) produced significantly greater increases in HDL-C than either the SIMVA (pooled) or the ERN/LRPT monotherapy groups. Individual dose comparisons yielded similar results. The greater HDL-C increases with ERN/LRPT+SIMVA versus the individual components were observed during the first four weeks of therapy (i.e. with the 1 g niacin dose). At 12 weeks, the observed increases in HDL-C were consistent with those reported for extended-release niacin.15 Apo A-I, the principal lipoprotein of HDL-C, was increased similarly in the ERN/LRPT 2 g+SIMVA (pooled) group and ERN/LRPT group relative to the SIMVA (pooled) monotherapy group.
Consistent with the LDL-C, HDL-C and TC findings, the percentage reductions in the ratios of LDL-C:HDL-C and TC:HDL-C were significantly greater in the ERN/LRPT 2 g+SIMVA (pooled) group than in either the SIMVA (pooled) or ERN/LRPT monotherapy groups. ERN/LRPT 2 g+SIMVA (pooled) also produced significantly larger decreases in TGs compared with either ERN/LRPT or pooled SIMVA monotherapy. Individual dose comparisons, as well as results following the first four weeks of treatment with ERN/LRPT 1 g+SIMVA (pooled), were consistent with these findings. Again, reductions in TG were consistent with published values for extended-release niacin in the literature.15
Lp(a) is believed to be a highly atherogenic lipoprotein and niacin is the only available agent that has been shown to reduce Lp(a) levels.16 As expected, ERN/LRPT, administered alone or with SIMVA, produced similar reductions of about 20% in Lp(a) and SIMVA alone had no effect on Lp(a). Despite the incremental reductions in LDL-C and increases in HDL-C produced by ERN/LRPT+SIMVA, the co-administration did not produce additional reductions in CRP beyond SIMVA monotherapy and there appeared to be no effect of ERN/LRPT on CRP levels. While CRP is a marker of inflammation, recognised as a risk factor for cardiovascular events independent of traditional risk factors, a clinical benefit specifically attributable to lowering CRP levels with any pharmacologic intervention has not been demonstrated. By comparison, niacin has been shown to reduce the risk of CHD in a large clinical outcomes study as well as slow the progression of atherosclerotic disease in angiographic and intima-media thickness (IMT) studies.17,18
The incidence of serious clinical AEs was low and comparable among treatment groups. The discontinuation rate was low in all seven treatment groups and primarily driven by flushing-related symptoms and gastrointestinal disorders in the ERN/LRPT treatment groups. Increases in hepatic transaminase and CK levels were evaluated carefully in view of the known effects of lipid-altering agents on these parameters.19-21 Only one case of hepatitis was observed in the ERN/LRPT 2 g + SIMVA group, which was ascribed to alcohol abuse and deemed not treatment related. The incidence of consecutive elevations in ALT and/or AST levels of at least 3 x ULN was low and similar across the treatment groups. There were no reports of myopathy or rhabdomyolysis and no differences in muscle-related AEs, either related or unrelated to treatment. The incidence of CK elevations was comparable across treatment groups. Although the small sample size in this study precludes definitive conclusions regarding important muscle-related AEs, the absence of a signal is consistent with the literature pertaining to the use of statins and niacin and the low risk of myopathy.19 Niacin has been shown to produce small increases in FSG in some patients and slightly worsen glycaemic control in patients with diabetes.19,22,23 Overall, AEs related to glycaemic control (diabetes mellitus, impaired fasting glucose, insulin resistance) were low and similar across treatment groups. The small median increases in FSG in both niacin-treated groups appeared to be consistent with the small effects on blood glucose observed with other niacin-based therapies.19,22,23
In summary, ERN/LRPT+SIMVA significantly improved the overall lipid profile relative to either agent alone and was generally well tolerated in patients with primary hypercholesterolaemia or mixed hyperlipidaemia. These data support the use of ERN/LRPT for use with a statin in the treatment of patients with dyslipidaemia.
Acknowledgement
Presented at the American Heart Association Scientific Sessions 2007, Orlando, FL, USA, November 4–7, 2007.
Conflict of interest
Funding Source: Merck & Co, Inc., Whitehouse Station, NJ, USA.Disclosures: GG, NL, ST-B, CMS, RCP, YM and JFP are employees of Merck & Co., Inc. and may hold stock/stock options in the company. CB was an investigator on this study and has received research grants and honoraria from Merck & Co., Inc.
Study investigators
Brazil: Fonseca FA; Saraiva JFK; Moriguchi EH; Bertolami MC; Feitosa GS; Canada: Ma PTS; Mymin D; Somani R; Shu D; Somani R; Keegan P; Denmark: Klausen IC; Stender S; Schmidt EB; France: Farnier M; Tondut J; Richter D; Malaysia: Sim KH; Chan SP; Mohamed M; Hong Kong: Tse HF; Tan KCB; Lithuania: Laucevicius A; Petrauskiene B; Rudys A; Celutkiene J; Brazdzionyte J; Vasiliauskas D; Maksvytis A; Kavaliauskiene R; Varonexkas G; Netherlands: Jonker JJC; Stalenhoef AFH; Norway: Ose L; Woie L; Peru: Rodriguez A; Segura L; Sanchez-Palacios MR; Poland: Kochmanski M; Pupek-Musialik D; Opolski G; Sweden: Hanning JP; Cherfan P; Ulvenstam G; Lindholm C-J; Noppa H; Holm D; Taiwan: Hou, J-YC; Wu T-C; Cheng S-M; United Kingdom: Sarmiento RA; Pitts C; Pawa R; Taylor SD; Shaw H; Pavel I; Khursheed M; Mohun S; Pavel I; Robinson JS; United States: Anastasi LJ; Arboleda V; Ashwal J; Ballantyne CM; Basista MP; Bays HE; Henry JGA; Bielski RJ; Bittar N; Brinton EA; Buchanan PP; Cheung DG; Bertini NA; Cohen SA; Cowan LI; Dionne DJ; Eder FS; Forker AD; Fraser NJ; Gassner LP; Gilderman LI; Harris LM; Isakov T; Kahn J; Kelly RL; Kerzner B; Kipnes MS; Kripsak JP; Lewin AJ; Littlejohn TW III; Conway MJ; Mangurson S; McCullough PA; McGowan MP; McKeown-Biagas CL; Mora YF; Murray AV; Niederman AL; Noss MJ; Odio AJ; Pierson M; Rosenblit PD; Sensenbrenner JW; Shapiro J; Sharp SC; Sideropoulos HP; Snyder BD; Strout CB; Topkis RL; Weerasinghe MU; Weinstein JR; Willis JG; Wilmer CI; Winslow DH; Borders JL; Burton R; Kamdar B; Short BH; Vanderneck CA.
Key messages
- Co-administration of niacin with a statin offers the potential for additional lipid management and cardiovascular risk reduction; however, sustained use of niacin is limited by flushing, mediated primarily by prostaglandin D2 (PGD2)
- A combination tablet containing 1 g of extended-release niacin and 20 mg of laropiprant (ERN/LRPT), a PGD2-receptor (DP1) antagonist, offers improved tolerability, supporting a simplified 1–2 g dosing regimen and improved adherence
- The current worldwide, multi-centre, randomised, double-blind, factorial design, 12-week parallel-group study assessed the efficacy and safety of ERN/LRPT co-administered with simvastatin in patients with primary hypercholesterolaemia or mixed hyperlipidaemia. The results show that ERN/LRPT + simvastatin significantly improved the overall lipid profile compared with ERN/LRPT and simvastatin alone and was generally well tolerated
References
- Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421.
- Cheng K, Wu TJ, Wu KK et al. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc Natl Acad Sci U S A 2006;103:6682–7.
- Birjmohun RS, Hutten BA, Kastelein JJ, Stroes ES. Increasing HDL cholesterol with extended-release nicotinic acid: from promise to practice. Neth J Med 2004;62:229–34.
- Mills E, Prousky J, Raskin G et al. The safety of over-the-counter niacin. A randomized placebo-controlled trial. BMC Clin Pharmacol 2003;3:4–11.
- Knopp RH, Ginsberg J, Albers JJ et al. Contrasting effects of unmodified and time-release forms of niacin on lipoproteins in hyperlipidemic subjects: clues to mechanism of action of niacin.Metabolism 1985;34:642–50.
- Kaijser L, Eklund B, Olsson AG, Carlson LA. Dissociation of the effects of nicotinic acid on vasodilatation and lipolysis by a prostaglandin synthesis inhibitor, indomethacin, in man. Med Biol1979;57:114–17.
- Sturino CF, O’Neill G, Lachance N et al. Discovery of a potent and selective prostaglandin D(2) receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]-acetic acid (MK-0524). J Med Chem 2007;50:794–806.
- Paolini JP, Mitchel YB, Reyes R et al. Effects of laropiprant on nicotinic acid-induced flushing in dyslipidemic patients. Am J Cardiol 2008;101:625–30.
- Maccubbin D, Bays HE, Olsson AG, et al. Lipid-modifying efficacy and tolerability of extended release niacin/laropiprant in patients with primary hypercholesterolaemia or mixed dyslipidaemia.Int J Clin Pract 2008;62:1959–70.
- The coronary drug project. JAMA 1972;221:918.
- Brown BG, Zhao XQ, Chait A et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001;345:1583–92.
- Brown BG, Hillger L, Zhao XQ, Poulin D, Albers JJ. Types of change in coronary stenosis severity and their relative importance in overall progression and regression in coronary disease. Observations from the FATS trial. Familial Atherosclerosis Treatment study. Ann NY Acad Sci 1995;748:407–17.
- The Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease. JAMA 1975;231:360–81.
- Canner PL, Berge KG, Wenger NK et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245–55.
- Guyton JR. Extended-release niacin for modifying the lipoprotein profile. Expert Opin Pharmacother 2004;5:1385–98.
- Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary artery disease. Meta-analysis of prospective studies. Circulation 2000;102:1082–5.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial biology for the investigation of the treatment effects of reducing cholesterol (ARBITER) 2. A double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004;110:3512–17.
- Mack WJ, LaBree L, Liu C-R, Liu C-H, Selzer RH, Hodis HN. Correlations between measures of atherosclerosis change using carotid ultrasonography and coronary angiography. Atherosclerosis2000;150:371–9.
- Guyton JR, Bays HE. Safety considerations with niacin therapy. Am J Cardiol 2007;99(Suppl):22C–31C.
- Tatò F, Vega GL, Grundy SM. Effects of crystalline nicotinic acid-induced hepatic dysfunction of serum low-density lipoprotein cholesterol and lecithin cholesteryl acyl transferase. Am J Cardiol1998;81:805–07.
- Rader JI, Calvert RJ, Hathcock JN. Hepatic toxicity of unmodified and time-release preparations of niacin. Am J Med 1992;92:77–81.
- Grundy SM, Vega GL, McGovern ME et al. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes – results of the assessment of diabetes control and evaluation of the efficacy of niaspan trial. Arch Intern Med 2002;162:1568–76.
- Elam MB, Hunninghake DB, Davis KB et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease. JAMA 2000;284:1263–70.
