A 58-year-old woman presented with a five-month history of epigastric pain, lower extremity oedema and orthopnoea. On examination she had postural hypotension, raised jugular venous pressure, hepatomegaly and pitting pedal oedema. Electrocardiogram (ECG) showed sinus tachycardia, low QRS voltage and a Q-wave in precordial leads V1–V4. A coronary angiogram was normal. IgG kappa monoclonal protein was detected in her serum and urine
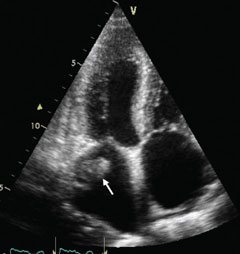
A transthoracic echocardiogram showed a large mobile mass 3.2 x 1.7 cm protruding from left atrial appendage, consistent with mural thrombus (figure 1). Concentric left ventricular wall and right ventricular free-wall thickening with a granular, ‘sparkling’ appearance of the myocardium was noticed. The left ventricle (LV) was globally hypokinetic with a reduced LV ejection fraction of 40%. There was thickening of the cardiac valves and atrial septum, dilatation of the atria, inferior vena cava and hepatic veins, with systolic flow reversals, and a small circumferential pericardial effusion. A restrictive LV filling pattern was apparent, as evidenced by E/A ratio 2.8, shortened deceleration time (120 ms), E/e’ ratio 27.5 and a predominantly diastolic pulmonary venous flow. There was only minimal atrial reversal in the pulmonary veins, a small mitral A wave, and no A’ visible on mitral annulus tissue Doppler, suggesting atrial electromechanical dissociation.
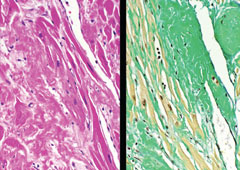
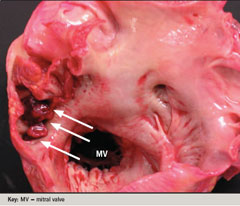
The patient was hospitalised for anticoagulation therapy with intravenous heparin. She became hypotensive and needed pressor support and ventilation. On the third hospitalisation day she died. An autopsy was performed and showed thrombi in the left atrial appendage (figure 2) and right atrial appendage. There were multiple infarctions in the small bowel, colon, kidneys and spleen. Microscopy demonstrated extensive amyloid deposition in the heart (figure 3), kidney, spleen, gastrointestinal tract and pancreas.
Discussion
The mechanism for intracardiac thrombosis in cardiac amyloidosis is unclear. Severely reduced atrial contractility secondary to amyloid infiltrate, increased left atrial afterload and left atrial enlargement with depressed LV systolic and diastolic function partially account for left atrial thrombosis in sinus rhythm. Vigilant screening for intracardiac thrombus by trans-oesophageal echocardiography may be indicated in patients with AL type of cardiac amyloidosis. In such patients, early anticoagulation might reduce morbidity and mortality related to thromboembolic phenomenon.
Conflict of interest
None declared.
