Cardiovascular disease is the biggest killer in the UK causing 198,000 deaths per year, and stroke is the most common cause of disability in women. Can individuals at increased risk be identified and can heart attacks and strokes be prevented?
Traditional methods of risk assessment for cardiovascular events use conventional risk factors to calculate risk often expressed as the 10-year Framingham Risk Score (10y FRS). However, these methods are far from perfect. Although they identify high-risk groups, if followed up these high-risk groups contain at best only a fraction of the events that will occur in the subsequent 10 years. In the Prospective Cardiovascular Munster (PROCAM) study 6.5% of the population were classified as high risk (10-year risk >20%), 14% as intermediate risk (10-year risk 10–20%) and 79.5% as low risk (10-year risk <10%). At 10 years, 33% of all the myocardial infarctions (MIs) occurred in the high-, 35% in the intermediate- and 32% in the low-risk group. This is because the low-risk group was very large and at least 30% of those who developed MI did not have any of the conventional risk factors.
Screening and improved selection of individuals for more effective prevention is now possible because:
(a) preclinical (silent) atherosclerotic plaques may develop in the arteries slowly over several decades before they rupture or obstruct an artery becoming clinically manifest
(b) screening methods are now available for detecting the presence and stability of such plaques
(c) current prophylaxis with aggressive risk factor modification can reduce morbidity and mortality from MI and stroke by 50%.
Two methods are currently popular: coronary artery calcium scoring (CACS) using multi-slice computed tomography (CT)-scanning known as electron beam tomography (EBT), and ultrasonic arterial scanning (figure 1). Both provide information that can improve the 10y FRS.
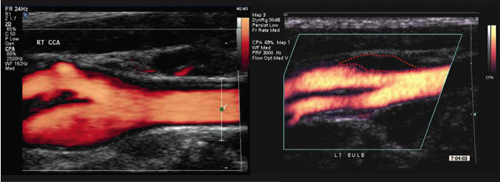
CACS using multi-slice
CT-scanning
Six prospective studies have demonstrated that CACS is an independent predictor of future coronary events,1 i.e. it provides information over and above that of the 10y FRS. The risk of annual fatal MI for CACS less than 100 is low (<0.4%), for CACS 100–400 it is moderate (1.3%) and for CACS 400 or higher it is high (2.4%). However, it cannot identify those with non-calcific unstable plaques, which are responsible for most heart attacks.1 Also, the finding of a high CACS in the absence of any significant coronary artery stenosis (>50%) is common, indicating the need to improve predictive ability.
On the basis of the available data the American College of Cardiology Foundation and American Heart Association have stated in their guidelines1 that screening is of limited value in individuals at low risk (10y FRS <10%). However, in individuals at intermediate risk (10y FRS 10–20%) the finding of CACS of 400 or higher would increase the risk to that noted with diabetes or peripheral arterial disease,2 altering clinical-decision making. Individuals with a high 10y FRS (≥20%) should be treated aggressively according to the current National Cholesterol Education Program (NCEP) III guidelines and do not require additional testing.2
A disadvantage of CACS is that it is expensive, and the high radiation dose associated with it does not allow repeated testing. Also, CACS does not provide information on stroke risk.
Screening with ultrasound
High-resolution ultrasound can provide images of the arterial wall and plaques with measurements of intima-media thickness (IMT), plaque thickness and area at a resolution of 0.2 mm, and plaque echodensity. Although IMT can be used to study the effect of risk factor modification in large groups, and has become a validated biomarker,3 it is only marginally better than conventional risk factors in identifying individuals at increased risk. However, new ultrasonic arterial wall measurements, such as the presence and thickness of plaques4-6 and plaque echolucency6-8 not only in the carotid but also in the common femoral artery, are stronger predictors of risk with a relative risk (RR) of 3.0 to 5.0.
Two prospective studies have shown that carotid plaque area is a better predictor of future MI than IMT.9,10 In the Tromsø study,9 IMT, total plaque area and plaque echolucency were measured in 6,226 men and women aged 25 to 84 years with no previous MI followed for six years. The adjusted RR (95% confidence interval [CI]) between the highest plaque area tertile versus no plaque was 1.56 (1.04 to 2.36) in men and 3.95 (2.16 to 7.19) in women. The adjusted corresponding RR (95% CI) for IMT was 1.73 (0.98 to 3.06) in men and 2.86 (1.07 to 7.65) in women. Plaque echolucency (low collagen content) indicating plaque instability was also associated with increased risk of MI. In the study performed in Canada,10 carotid plaque areas from 1,686 individuals followed for up to five years were categorised into four quartiles. The combined five-year risk of stroke, MI and vascular death by quartiles of plaque area was: 5.6%, 10.7%, 13.9% and 19.5%. Thus, the presence, size and type of preclinical plaques are emerging as having a strong association with coronary heart disease and stroke. They can be used to re-classify those in intermediate and low 10y FRS groups into higher or lower risk.
In our own ongoing study, 2,000 individuals over the age of 40 are being screened for conventional risk factors, clinical and preclinical cardiovascular disease and followed-up for five years.11 Both carotid and both common femoral bifurcations are scanned with ultrasound. In the low 10y FRS group, 42% of asymptomatic individuals have atherosclerotic plaques. In another recent study using ultrasound, carotid plaques were found in 34% of individuals at low 10y FRS and CACS of zero.12
Advantages of screening with ultrasound are the relatively low cost and absence of radiation. It can be performed in 30 minutes. In addition, it can be repeated at six-monthly intervals or annually providing information on plaque progression or regression. An added benefit of ultrasound is that with the addition of an extra 5–10 minutes, men over the age of 65 years can be screened for the presence of abdominal aortic aneurysm, as recently recommended by the National Institute for Health and Clinical Excellence (NICE).
A rational screening plan
Individuals with low 10y FRS: should be screened with ultrasound. Absence of plaques, found in 60% of individuals in this low-risk group, will confirm the low risk and further follow-up with ultrasound will not be necessary for three to four years. However, the presence of plaques, found in the other 40%, will result in re-classification to a higher risk and will prompt the clinician, not only to advise on risk factor modification, but also to look for non-conventional risk factors, such as elevated homocysteine. Two recent prospective randomised-controlled trials in individuals with known cardiovascular disease have demonstrated that lowering homocysteine with vitamin supplements has reduced the risk of ischaemic stroke by 25%.13,14
Individuals with an intermediate 10y FRS: should be initially screened with ultrasound. Those with plaques are advised to have aggressive risk factor modification. They are told that plaques should not be allowed to progress and that regression, which with treatment to target can occur in 28%, is associated with a 50% reduction in risk.10 They are also advised to have an annual electrocardiogram (ECG) stress test. If plaques are absent then CACS may be performed. The latter will allow re-classification to a lower- or higher-risk group with confidence.
Individuals with a high 10y FRS: are advised to have aggressive risk factor modification according to the current guidelines. Screening with ultrasound in order to follow plaque progression or regression is optional. However, this and the associated plaque images provide a strong incentive to persevere with prophylactic therapy as compliance can be challenging.
The strategy outlined above uses a combination of conventional risk factors with ultrasound, which is in the forefront of non-invasive, inexpensive imaging modalities for screening asymptomatic individuals. It should go a long way towards achieving the government target of reducing heart attacks and strokes by 40%15 because it identifies many individuals at increased risk that would be missed by 10y FRS.
Setting up cardiovascular screening services or centres throughout the country should be seriously considered. The major investment would not be in the equipment, which is now relatively inexpensive and portable, but in trained staff. Asymptomatic individuals should be referred to such centres by their own physicians. This should avoid inappropriate use of the services. The referring doctor should be responsible for making the decisions on targeted preventive measures based on the individual’s clinical and imaging assessment.
A final consideration is that ultrasound could prove a potent tool in lifestyle
modification, as there is nothing more powerful than asymptomatic individuals
experiencing a real-time image of their arteries showing atherosclerotic deposits.
Such deposits are the end result of all risk factors, known, emerging and yet
unknown including genetic.
Conflict of interest
None declared.
References
1. Greenland P, Bonow RO, Brundage BH et al. ACCF/AHA expert consensus document on coronary artery calcium scoring. Circulation 2007;115:402–26.
2. Third report of the NCEP Expert Panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421.
3. Lorenz MW, Marcus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness. Circulation 2007;115:459–67.
4. Ebrahim S, Papacosta O, Whincup P et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke 1999;30:841–50.
5. Hollander M, Bots ML, Iglesias del Sol A et al. Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly. The Rotterdam Study. Circulation 2002;105:2872–7.
6. Schmidt C, Fagerberg B, Hulthe J. Non-stenotic echolucent ultrasound-assessed femoral artery plaques are predictive for future cardiovascular events in middle-aged men. Atherosclerosis 2005;181:125–30.
7. Honda O, Sugiyama S, Kugiyama K et al. Echolucent carotid plaques predict future coronary events in patients with coronary artery disease. J Am Coll Cardiol 2004;43:177–84.
8. Seo Y, Watanabe S, Ishizu T et al. Echolucent carotid plaques as a feature in patients with acute coronary syndrome. Circ J 2006;70:1629–34.
9. Johnsen SH, Mathiesen EB, Joakimsen O et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow up study of 6226 persons: the Tromsø study. Stroke 2007;38:2873–80.
10. Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. A tool for targeting and evaluating vascular preventive therapy. Stroke 2002;33:2916–22.
11. Griffin M, Nicolaides AN, Tyllis TH et al. Carotid and femoral arterial wall changes and the prevalence of clinical cardiovascular disease. Vascular Medicine 2009;14:227–32.
12. Lester SJ, Eleid MF, Khandheria BK, Hurst RT. Carotid IMT thickness and coronary artery calcium score as indications of subclinical atherosclerosis. Mayo Clin Proc 2009;84:229–33.
13. Lonn E, Yusuf S, Arnold JM et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med 2006;354:1567–77.
14. Saposnik G, Ray JG, Sheridan P, McQueen M, Lonn E. Homocysteine-lowering therapy and stroke risk, severity and disability. Stroke 2009;40:1365–72.
15. Department of Health. Coronary heart disease national service frameworks. London: DoH, 2000.
