Outcome in patients with peripartum cardiomyopathy (PPCM) is variable. Recovery of left ventricular function is observed in between 23% and 51% of cases at six months after diagnosis. Despite standard medical therapy, both morbidity and mortality remain high. Recent evidence has suggested that dopamine-receptor agonists may be beneficial in the treatment of this condition. We describe a case of a patient with PPCM who developed rapid normalisation of left ventricular function following addition of carbergoline, a long-acting dopamine-receptor agonist, to her conventional heart failure therapy.
Case report
A 25-year-old primigravida with a twin pregnancy was admitted with eclampsia at 37 weeks’ gestation following a brief, witnessed, tonic-clonic seizure at home. Prior to admission her pregnancy had been uncomplicated with normal blood pressure (BP) recordings throughout. Her past medical history was unremarkable and there was no family history of cardiomyopathy. On arrival at the emergency department, she was unconscious (Glasgow coma scale [GCS] 7/15) and markedly hypertensive (BP 170/100 mmHg) with 3+ proteinuria on urinalysis. Physical examination revealed normal cardiovascular, respiratory and abdominal systems. Following administration of intravenous magnesium sulphate, her BP improved. She was intubated and ventilated, and emergency caesarean section was performed with delivery of two healthy twin babies. Her BP normalised and she experienced no further seizures. She was extubated on the second day of admission.
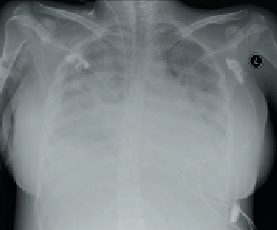
On the third day of admission, she experienced dyspnoea, orthopnoea and cough. Physical examination revealed normal BP, sinus tachycardia (110 beats per minute), raised jugular venous pressure, lung crackles on chest auscultation and bilateral pedal oedema. An electrocardiogram (ECG) confirmed sinus tachycardia with no ST-segment changes. Routine blood investigations including full blood count, blood sugar, renal profile and liver function tests were all within normal range. However, her cardiac enzymes were mildly elevated with creatinine kinase 317 U/L (24–173) and troponin T 0.18 ug/L (0–0.10). Chest radiography revealed pulmonary congestion (figure 1).
A transthoracic echocardiogram performed the same day revealed a normal-sized left ventricle (left ventricular end diastolic [LVED] diameter 4.3 cm, left ventricular end systolic [LVES] diameter 3.5 cm) with severe impairment of systolic function (ejection fraction 25%) (figure 2). There was severe hypokinesia of the posterior wall and apical segments. No valvular disease or left ventricular hypertrophy was detected. The patient was treated with an intravenous diuretic (frusemide) and an angiotensin-converting enzyme (ACE) inhibitor (enalapril) with subsequent clinical improvement. A beta blocker (bisoprolol) was added once her condition had stabilised.
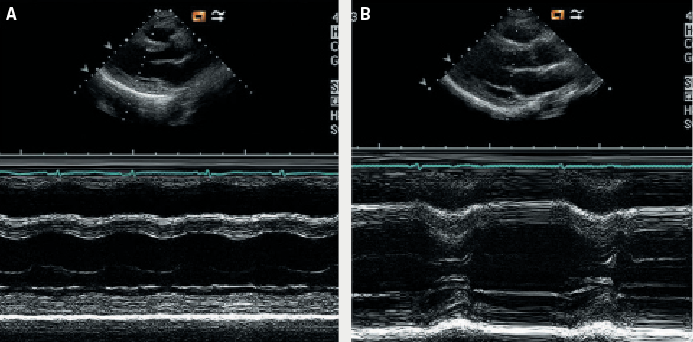
Further laboratory investigations included negative viral and auto-antibody screens. Breastfeeding was discouraged in view of her pharmacotherapy and the possibility of mother-to-baby drug transmission. Since the patient was not lactating, she was administered a long-acting dopamine-receptor agonist, oral cabergoline (0.25 mg twice daily for two days), to stop breast milk production and prevent uncomfortable breast engorgement. This treatment was initiated 48 hours after the onset of her cardiac symptoms. The patient remained well during the remainder of her admission. Attempts at increasing her medication as prophylaxis against heart failure resulted in the development of hypotension, and so she was eventually discharged on frusemide 40 mg once daily, enalapril 5 mg once daily and bisoprolol 1.25 mg once daily. A repeat echocardiogram two weeks postpartum revealed that her left ventricular function had normalised (ejection fraction 79%) with complete resolution of the previously documented regional wall motion abnormalities. She remained asymptomatic and continued on the same doses of ACE inhibitor and beta blocker treatment.
Discussion
PPCM is a rare cardiomyopathy that can affect women at any time from the last month of pregnancy up until five months after delivery. Diagnosis requires documentation of poor left ventricular function in the absence of other identifiable causes of heart failure.1 The precise aetiology of PPCM is unknown but there is some evidence to implicate an underlying myocarditis. O’Connell et al. observed that 29% of patients with PPCM had myocarditis confirmed by endomyocardial biopsy, in comparison to only 9% of patients with idiopathic dilated cardiomyopathy (DCM).2 In another study by Midei et al., 14 of 18 patients with PPCM were demonstrated to have histologically proven myocarditis.3 Several triggers for myocyte injury have been postulated including viral infection, autoimmune disease, hormonal changes, genetic disorders or toxaemia.4,5
Our patient developed heart failure three days postpartum, which is in keeping with PPCM. This diagnosis is further supported by echocardiographic demonstration of depressed left ventricular function in the absence of other identifiable causes of heart failure. The patient had established risk factors for the development of PPCM including multiple pregnancy and pre-eclampsia.
We do not believe that severe hypertension was contributory to her heart failure since it was transient and she was normotensive in the period preceding her decompensation. Although neurogenic stunned myocardium is a well-established complication of central nervous system disorders, this clinical entity has most commonly been reported with intractable seizures due to status epilepticus.6,7 It is unlikely that the single, self-limiting seizure in our case would have produced a sufficient catecholamine surge to cause such profound myocardial stunning. Furthermore, presentation of neurogenic stunned myocardium typically mimics acute myocardial infarction and manifests as Takotsubo cardiomyopathy, whereas our patient had no evidence of ECG abnormalities or apical ballooning to corroborate this.
The prognosis of patients with PPCM is difficult to predict, with published rates of left ventricular function recovery varying between 23% and 51%.8,9 With standard medical treatment, improvement in left ventricular function is typically observed to occur after at least six months of diagnosis.10 The mainstay of treatment is similar to other forms of cardiomyopathy, namely ACE inhibitor (or angiotensin-receptor blocker), beta blockers, aldosterone antagonist, diuretics and digoxin.
There have been several encouraging reports regarding the use of bromocriptine, a short-acting dopamine-receptor agonist, in PPCM.11,12 More recently, a proof-of-concept pilot study has shown that addition of bromocriptine to standard heart failure therapy improved left ventricular ejection fraction in a small cohort of women with newly diagnosed PPCM.13 Bromocriptine inhibits the release of prolactin, which is secreted from the anterior pituitary gland during pregnancy and postpartum period and stimulates lactation. Animal experiments have shown that prolactin is cleaved into a 16 kDa form as a result of oxidative stress. The 16 kDa prolactin is both anti-angiogenic and pro-apoptotic and may, therefore, mediate PPCM.14 In keeping with this theory, Hilfiker-Kleiner et al. demonstrated that forced generation of 16 kDa prolactin induces a PPCM phenotype in mice.15 Furthermore, treatment with bromocriptine prevented development of PPCM in this murine model. Inhibition of prolactin secretion may, therefore, constitute a novel therapeutic strategy for women with this condition.
Our patient’s symptoms were initially relieved by standard heart failure treatment consisting of diuretics and ACE inhibition with subsequent addition of a beta blocker. However, the rapid normalisation of the left ventricular function observed within two weeks of PPCM diagnosis has never previously been described on this conventional therapy. The low doses of ACE inhibitor and beta blocker treatment administered would be unlikely to account for the dramatic change in left ventricular function in such a short time interval. We, therefore, suggest that the introduction of cabergoline contributed to the rapid improvement in left ventricular function observed.
Both bromocriptine and cabergoline are ergoline derivatives but, unlike bromocriptine, cabergoline has an extremely long half-life (c. 65 hours) and is highly selective for the dopamine D2-receptor. Although several reports have documented the potential efficacy of bromocriptine in PPCM, to the best of our knowledge there is only one other published case report that describes the use of cabergoline.16 However, in contrast to that report, where only partial left ventricular recovery was observed, cabergoline treatment in our patient was associated with complete normalisation of left ventricular function.
Conclusion
Our case illustrates the potential therapeutic benefit of cabergoline, a dopamine-receptor agonist, as an adjunct to facilitate early recovery of left ventricular function in PPCM. Whether or not this will translate to an improvement in the overall prognosis of patients with PPCM remains to be seen. In view of the rarity of this condition, a collaborative multi-centre research strategy is required to further evaluate the efficacy of carbergoline therapy.
Conflict of interest
AG recieves salary support from CORDA.
References
- Pearson GD, Veille JC, Rahimtoola S et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA 2000;283:1183–8. (doi: 10.1001/jama.283.9.1183)
- O’Connell JB, Costanzo-Nordin MR, Subramaniam R et al. Peripartum cardiomyopathy: clinical, hemodynamic, histologic and prognostic characteristic. J Am Coll Cardiol 1986;8:52–6. (doi: 10.1016/S0735-1097(86)80091-2)
- Midei M, DeMent S, Feldman A, Hutchins G, Baughman K. Peripartum myocarditis and cardiomyopathy. Circulation 1990;81:922–8. (doi: 10.1161/01.CIR.81.3.922)
- Ntusi NBA, Mayosi BA. Aetiology and risk factors of peripartum cardiomyopathy: a systematic review. Int J Cardiol 2009;131:168–79. (doi: 10.1016/j.ijcard.2008.06.054)
- Sanderson JE, Olsen EG, Gatei D. Peripartum heart disease: an endomyocardial biopsy study. Br Heart J 1986;56:285–91. (doi: 10.1136/hrt.56.3.285)
- Shimizu M, Kagawa A, Takano T, Masai H, Miwa Y. Neurogenic stunned myocardium associated with status epilepticus and postictal catecholamine surge. Intern Med 2008;47:269–73. (doi: 10.2169/internalmedicine.47.0499)
- Lemke DM, Hussain SI, Wolfe TJ et al. Tako-Tsubo cardiomyopathy associated with seizures. Neurocrit Care 2008;9:112–17. (doi: 10.1007/s12028-008-9075-x)
- Sliwa K, Foster O, Libhaber E et al. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J 2006;27:441–6. (doi: 10.1093/eurheartj/ehi481)
- Hu CL, Li YB, Zou YG et al. Troponin T measurement can predict persistent left ventricular dysfunction in peripartum cardiomyopathy. Heart 2007;93:488–90. (doi: 10.1136/hrt.2006.087387)
- Sliwa K, Skudicky D, Bergemann A, Candy G, Puren A, Sareli P. Peripartum cardiomyopathy: analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/APO-1. J Am Coll Cardiol 2000;35:701–05. (doi: 10.1016/S0735-1097(99)00624-5)
- Hilfiker-Kleiner D, Meyer GP, Schieffer E et al. Recovery from postpartum cardiomyopathy in 2 patients by blocking prolactin release with bromocriptine. J Am Coll Cardiol 2007;50:2354–5. (doi: 10.1016/j.jacc.2007.10.006)
- Habedank D, Kuhnle Y, Elgeti T, Dudenhausen JW, Haverkamp W, Dietz R. Recovery from peripartum cardiomyopathy after treatment with bromocriptine. Eur J Heart Fail 2008;10:1149–51. (doi: 10.1016/j.ejheart.2008.09.001)
- Sliwa K, Blauwet L, Tibazarwa K et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation 2010;121:1465–73. (doi: 10.1161/CIRCULATIONAHA.109.901496)
- Hilfiker-Kleiner D, Schieffer E, Meyer GP, Podewski E, Drexler H. Postpartum cardiomyopathy. Dtsch Arztebl Int 2008;105:751–6.
- Hilfiker-Kleiner D, Kaminski K, Podewski E et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007;128:589–600. (doi: 10.1016/j.cell.2006.12.036)
- De Jong JSSG, Rietveld K, van Lochem LT, Bouma BJ. Rapid left ventricular recovery after cabergoline treatment in a patient with peripartum cardiomyopathy. Eur J Heart Fail 2009;br 11:220–2. (doi: 10.1093/eurjhf/hfn034)
