A 47-year-old man with known bicuspid aortic valve was admitted with a six-week history of night sweats and malaise. Clinically, aortic systolic and diastolic murmurs were present. Temperature was 38ºC, white cell count was elevated at 13.8 x 109/L, erythrocyte sedimentation rate (ESR) was 44 mm/hr, and three consecutive blood cultures grew Streptococcus parasanguinis. Transthoracic (TTE), then transoesophageal (TOE) echocardiography was performed.
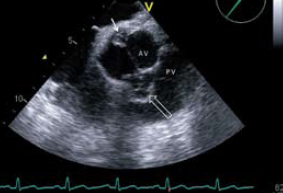
TOE confirmed a 0.8 cm vegetation at the posteriorcommissure of the aortic valve with an ill-defined, 1.6 cm diameter, loculated lesion anterior to the valve pressing into the right ventricular outflow tract. This was felt to be an abscess cavity (hollow arrow, figure 1). TOE showed no involvement or impairment of flow throughout the length of the left main stem (LMS) or proximal right coronary arteries (LMS is seen at 3 o’clock in figure 1). Colour Doppler assessment showed mild aortic stenosis and eccentric aortic regurgitation.
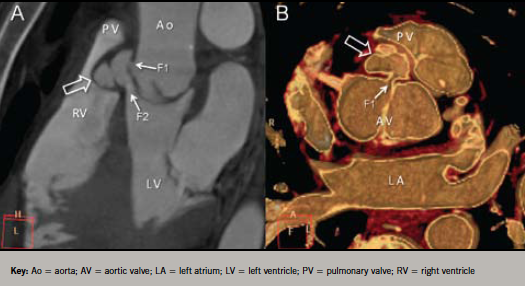
A 64-slice multi-detector cardiac computed tomography (64-MDCT) examination was performed to confirm diagnosis and assess coronary vessels prior to surgery. The study revealed the presence of an aortic root fistula (communication between two neighbouring cavities through an abscess). The abscess communicated with the aortic root and the left ventricular outflow tract (LVOT) (F1 and F2, respectively, in figure 2). The coronary arteries were normal. The patient successfully underwent urgent surgery.
Discussion
Abscess formation is a feared complication of infective endocarditis. It increases mortality and is an indication for surgical intervention. TTE is relatively poor at detection with sensitivity of only 28%; TOE is superior (87%) and is generally considered to be the investigation of choice.1 Both investigations should, however, be performed where abscess is suspected, as TTE may yield better images of an anterior root abscess, while TOE is better for posterior root abscess. TOE can also define the origins of the coronary arteries in relation to the abscess.
Colour Doppler is often helpful in demonstrating abnormal flow through an abscess or fistula. In this case, the abnormal flow associated with mixed aortic valve disease masked any jets associated with the fistula.
Cardiac computed tomography (CT) has evolved as a powerful tool in the assessment of coronary artery disease, but it may prove to have a useful role in the investigation of some cases of infective endocarditis, especially where abscess is suspected.2,3 CT, however, faces several challenges:
- Although spatial resolution is excellent (<1 mm), temporal resolution does not match TOE, and pyrexic patients may exhibit tachycardia.
- Only a few time points in the cardiac cycle are imaged, typically 40%, 70% and 75% of the R to R interval. Thus, appreciation of moving structures may be poor.
- The valve leaflets are both thin and mobile, and, therefore, difficult to image well – valve perforations can easily be missed.
Advantages of CT include:
- Multi-planar reconstruction allows ready appreciation of complex 3D structures such as the fistula seen in this case. Infiltration of infection into the myocardium is easy to see.
- CT offers the ability to image the entire coronary tree non-invasively, since catheter angiography runs the risk of septic embolisation in aortic valve endocarditis.4
In a head-to-head comparison of CT with TOE in infective endocarditis, both correctly identified 96% of vegetations (26 out of 27 patients) proven at surgery, while CT detected 100% of abscesses proven at surgery (nine out of nine patients) versus 89% for TOE (eight out of nine patients). The majority of vegetations missed by CT were ≤4 mm in size.5
European Cardiac Society Guidelines on infective endocarditis (2009) state that: “multi-slice CT has recently been shown to give good results in the evaluation of infective endocarditis (IE)-associated valvular abnormalities, as compared with TOE, particularly for the assessment of the perivalvular extent of abscesses and pseudoaneurysms”.6 We believe that the high spatial resolution of CT makes it most useful in aortic valve endocarditis, where it allows multi-planar and 3D imaging of complex perivalvular disease, and it may also avoid the embolic risk associated with invasive coronary angiography in this setting.
Conflict of interest
None declared.
Editors’ note
See also the Editorial by Susanna Price on pages 7–9 of this issue.
References
- Daniel WG, Mugge A, Martin RP et al. Improvement in the diagnosis of abscess associated with endocarditis by transoesophageal echocardiography. N Engl J Med 1991;324:795–800. http://dx.doi.org/10.1056/NEJM199103213241203
- McWilliams ET, Yavari A, Raman V. Aortic root abscess: multimodality imaging with computed tomography and gallium-67 citrate single-photon emission computed tomography/computed tomography hybrid imaging. J Cardiovasc Comput Tomogr 2011;5:122–4. http://dx.doi.org/10.1016/j.jcct.2010.10.004
- Gahide G, Bommart S, Demaria R et al. Preoperative evaluation in aortic endocarditis: findings on cardiac CT. Am J Roentgenol 2010;194:574–8. http://dx.doi.org/10.2214/AJR.08.2120
- Lentini S, Monaco F, Tancredi F, Savasta M, Gaeta R. Aortic valve infective endocarditis: could multi-detector CT scan be proposed for routine screening of concomitant coronary artery disease before surgery? Ann Thorac Surg 2009;87:1585–7. http://dx.doi.org/10.1016/j.athoracsur.2008.09.015
- Feuchtner GM, Stolzmann P, Dichtl W et al. Multislice computed tomography in infective endocarditis: comparison with transoesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009;53:436–44. http://dx.doi.org/10.1016/j.jacc.2008.01.077
- The task force on the prevention, diagnosis, and treatment of infective endocarditis of the European Society of Cardiology (ESC). Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009). Eur Heart J 2009;30:2369–413. http://dx.doi.org/10.1093/eurheartj/ehp285
