The global epidemic of obesity and type 2 diabetes, largely due to overconsumption and sedentary lifestyle, is a major challenge facing clinicians. In the UK, as in the European Region, the prevalence of obesity is rapidly increasing, highlighting a growing health challenge.1 In England (2003 data), 65% of males and 55% of females aged 16 years or more are either overweight or obese.1 As a consequence, the prevalence of the metabolic syndrome, of which dyslipidaemia (elevated triglycerides and low plasma levels of high-density lipoprotein [HDL] cholesterol) and central obesity are key features,2 is increasing.
Therapeutic lifestyle intervention underpins the management of dyslipidaemia, especially that associated with the metabolic syndrome. Indeed, the recent European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines on dyslipidaemia place emphasis on nutritional approaches, either alone or complementary to pharmacotherapy, in managing hypercholesterolaemia to reduce cardiovascular risk.3 This is clearly supported by concordant evidence from observational studies and randomised trials showing associations between lifestyle factors, effects on lipids and other biomarkers and cardiovascular disease risk. In the case–control INTERHEART study, involving 52 countries worldwide, four out of the nine major factors determining risk for myocardial infarction (MI) were lifestyle components.4 Three of these were protective, namely, physical activity, intake of fruit and vegetables and alcohol use, while smoking increased risk. Additionally, the other factors in the INTERHEART study associated with cardiovascular risk were also influenced by lifestyle: abdominal obesity, blood pressure, lipids, diabetes and stress. Cardiovascular risk increased cumulatively with an increasing number of risk factors. Clearly, targeting lifestyle factors should be fundamental to control of lipids and prevention of cardiovascular disease.
Diet
Dietary intervention favourably influences lipids and cardiovascular risk and should, therefore, be a cornerstone of treatment efforts. Observational studies have shown that traditional Mediterranean diets, including vegetables, fruits, fish, wholegrain cereals, legumes, unsaturated fats, moderate alcohol intake and limited consumption of red meat, improved lipids and glycaemic control and reduced cardiac mortality.5-7 The Lyon Diet Heart study8 tested whether converting to a Mediterranean diet, as well as substituting omega-3 canola oil and spread for added fats, could impact coronary event rates in MI survivors. Despite a similar coronary risk factor profile, subjects following the Mediterranean-style diet had significant and substantial reductions in coronary events (by at least 50%), as well as all-cause mortality, leading to early termination of the trial. It is a salient point that across the UK, two-thirds of adults do not consume the recommended five portions of fruit and vegetables a day.9
Improving the diet by substitution of saturated fats with unsaturated or monosaturated fats not only lowers low-density lipoprotein (LDL) cholesterol, but also benefits triglycerides and HDL cholesterol. For example, the PREDIMED study showed that a Mediterranean-style diet (including olive oil) raised HDL cholesterol (by 0.62 mmol/L) and lowered triglycerides (by 0.03 mmol/L), compared with a low-fat diet.7 This dietary approach is clearly valuable for targeting patients with the metabolic syndrome. Recent guidelines recommend that saturated fat intake should be less than 10% of the total caloric intake, and even lower in patients with hypercholesterolaemia.3 Trans-fatty acids, present in hydrogenated fats, should be eliminated from the diet as these have unfavourable effects on LDL cholesterol, triglycerides and HDL cholesterol.10
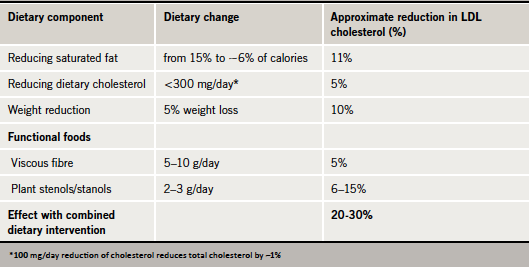
There is growing support for the value of so-called ‘functional foods’ in the diet, as incorporated in recent ESC/EAS guidelines.3 Consumption of plant sterols or stanols is consistently associated with lowering of LDL cholesterol levels by 7–10%.3 Additionally, increasing intake of soluble (viscous) fibre, such as in oats, can produce modest reductions in total and LDL cholesterol (by about 0.13 mmol/L).11 Overall, the combined effect of dietary changes can produce up to a 20–30% reduction in LDL cholesterol levels (table 1).12-16
Physical activity
Exercise is also a cornerstone for improving lipids, treating metabolic syndrome and reducing cardiovascular risk. While physical activity improves LDL cholesterol levels, there is also benefit in terms of lowering triglycerides and raising levels of HDL cholesterol.3 Not all of the effect of exercise is due to exercise-mediated weight loss, as there is evidence that both weight loss and physical exercise act independently to improve lipids and cardiovascular risk.17,18 Indeed, gaining weight or becoming physically inactive are among the main determinants for developing the metabolic syndrome.19 Increasing the amount and intensity of exercise have all been linked with benefit on lipids and lipoproteins.19,20
Implementation and adherence issues
Therapeutic lifestyle intervention is clearly effective in managing dyslipidaemia to reduce cardiovascular risk. Despite this, clinicians and patients fail to do their best with lifestyle intervention. First, how lifestyle advice is implemented and monitored by clinicians is often less than ideal, as illustrated by EURIKA (European Study on Cardiovascular Risk Prevention and Management in Daily Practice), a pan-European primary prevention study. Although advice on a healthy diet (low in fat and rich in vegetables and fruit) was provided to the vast majority (>80%) of patients, only one-half received written dietary advice and only one-third were referred to a dietitian.21 For patients, long-term adherence is the main issue. For example, in the Atherosclerosis Risk in Communities study including 12,744 subjects initially free of cardiovascular disease, only 0.1% of subjects maintained an ideal healthy lifestyle in the long-term.22 We need to address issues with non-adherence to optimise the benefit of lifestyle intervention.
The difficulties associated with maintaining a healthy lifestyle in the long term often mean that dyslipidaemic patients will require additional therapeutic intervention. Statin treatment remains the cornerstone of lipid-modifying treatment to prevent cardiovascular disease.23 Consideration may be given to the need for additional treatment for lowering elevated triglycerides or raising low HDL cholesterol. Once again, non-adherence to treatment is a key issue, especially in the high-risk patient. In a study reviewing adherence with three treatments prescribed in the post-MI setting (statin, beta blocker and aspirin), one in five patients stopped one medication and one in eight stopped all three treatments within one month of discharge from hospital.24 Furthermore, as is the case with all long-term therapy, there is progressive decline in adherence. In one study, less than half (~40%) of acute coronary syndrome patients were still taking statin therapy two years post-MI.25 This in turn translates to poorer outcomes.26
Promoting a healthy lifestyle and improving adherence to lipid-modifying treatments should be priorities for improving outcome. This is especially relevant in the light of increasing numbers of individuals at risk of coronary heart disease and ever increasing economic restraint in healthcare budgets.
Key messages
- Therapeutic lifestyle intervention underpins the management of dyslipidaemia
- Functional foods also have a role in management of elevated LDL cholesterol
- Metabolic syndrome is reversible with targeted intervention
- Improving adherence with lifestyle intervention and pharmacotherapy is a priority
References
- Branca F, Nikogosian H, Lobstein T (eds). The challenge of obesity in the WHO European Region andthe strategies for response. Summary. Copenhagen: WHO Regional Office for Europe, 2007. Available from: http://www.euro.who.int/__data/assets/pdf_file/0010/74746/E90711.pdf [accessed 17 December 2011].
- International Diabetes Federation. IDF worldwide definition of the metabolic syndrome. Available from: http://www.idf.org/metabolic-syndrome [accessed 17 December 2011].
- Reiner Z, Catapano AL, De Backer G et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769–818. http://dx.doi.org/10.1093/eurheartj/ehr158
- Yusuf S, Hawken S, Ounpuu S et al.; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52. http://dx.doi.org/10.1016/S0140-6736(04)17018-9
- Trichopoulou A, Bamia C, Trichopoulous D. Mediterranean diet and survival among patients with coronary heart disease in Greece. Arch Intern Med 2005;165:929–35. http://dx.doi.org/10.1001/archinte.165.8.929
- Giugliano D, Esposito K. Mediterranean diet and metabolic diseases. Curr Opin Lipidol 2008;19:63–8.
- Estruch R, Martinez-Gonzalez MA, Corella D et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med 2006;145:1–11.
- de Lorgeril M, Renaud S, Mamelle N et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994;343:1454–9. http://dx.doi.org/10.1016/S0140-6736(94)92580-1
- Heart UK. Cholesterol and a healthier nation: shared responsibility for better public health. Available at: http://www.nelm.nhs.uk/en/NeLM-Area/News/2011—December/15/Heart-UK-report-Cholesterol-and-a-healthier-nation–shared-responsibility-for-better-public-health/ [accessed 4 January 2012].
- Mensink RP, Katan MB. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N Engl J Med 1990;323:439–45. http://dx.doi.org/10.1056/NEJM199008163230703
- Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 1999;69:30–42.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421.
- Ginsberg HN, Kris-Etherton P, Dennis B et al. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects. The Delta Study, Protocol 1. Arterioscler Thromb Vasc Biol 1998;18:441–9. http://dx.doi.org/10.1161/01.ATV.18.3.441
- Howell WH, McNamara DJ, Tosca MA, Smith BT, Gaines JA. Plasma lipid and lipoprotein responses to dietary fat and cholesterol: a meta-analysis. Am J Clin Nutr 1997;65:1747–64.
- Wood PD, Stefanick ML, Williams PT, Haskell WL. The effects on plasma lipoproteins of a prudent weight-reducing diet, with or without exercise, in overweight men and women. N Engl J Med 1991;325:461–6. http://dx.doi.org/10.1056/NEJM199108153250703
- Van Gaal LF, Mertens IL, Ballaux D. What is the relationship between risk factor reduction and degree of weight loss? Eur Heart J 2005;7(suppl L):L21–L26. http://dx.doi.org/10.1093/eurheartj/sui082
- Kelley GA, Kelley KS, Roberts S, Haskell W. Efficacy of aerobic exercise and a prudent diet for improving selected lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. BMC Medicine 2011;9:74. http://dx.doi.org/10.1186/1741-7015-9-74
- Ekblom-Bak E, Hellenius ML, Ekblom O, Engstrom LM, Ekblom B. Fitness and abdominal obesity are independently associated with cardiovascular risk. J Intern Med 2009;266:547–57. http://dx.doi.org/10.1111/j.1365-2796.2009.02131.x
- Wannamethee SG, Shaper AG, Whincup PH. Modifiable lifestyle factors and the metabolic syndrome in older men: effects of lifestyle changes. J Am Geriatr Soc 2006;54:1909–14. http://dx.doi.org/10.1111/j.1532-5415.2006.00974.x
- Kraus WE, Houmard JA, Duscha BD et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med 2002;347:1483–92. http://dx.doi.org/10.1056/NEJMoa020194
- Banegas JR, López-García E, Dallongeville J et al. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: the EURIKA study. Eur Heart J 2011;32:2143–52. http://dx.doi.org/10.1093/eurheartj/ehr080
- Folsom AR, Yatsuya H, Nettleton JA et al.; ARIC Study Investigators. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011;57:1690–6. http://dx.doi.org/10.1016/j.jacc.2010.11.041
- National Institute for Health and Clinical Excellence. Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease [CG67]. London: NICE, 2008. Available from: http://www.nice.org.uk/nicemedia/pdf/CG67NICEguideline.pdf [accessed 4 January 2012].
- Ho PM, Spertus JA, Masoudi FA et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med 2006;166:1842–7. http://dx.doi.org/10.1001/archinte.166.17.1842
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002;288:462–7. http://dx.doi.org/10.1001/jama.288.4.462
- Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA 2007;297:177–86. http://dx.doi.org/10.1001/jama.297.2.177
Case scenario
Consider secondary causes of dyslipidaemia
 Hazel, a 49-year-old teaching assistant, was referred to the lipid clinic following admission with acute chest pain (subsequently considered non-cardiac, cardiac troponin negative). Her total cholesterol at the time of admission was 10.0 mmol/L. She had previously undergone thyroidectomy and radio-iodine ablation for papillary thyroid cancer. She had a history of treated hypertension (blood pressure [BP] 126/80 mmHg), was overweight (body mass index [BMI] 30.1 kg/m2), physically active, a moderate drinker (10 units per week), recent ex-smoker and dietary assessment indicated scope for improvement. Both parents had type 2 diabetes and hypercholesterolaemia but no clinical cardiovascular disease. She was discharged on simvastatin 40 mg/day, aspirin 75 mg/day, levothyroxine 125 µg/day and lisinopril 5 mg/day. There was no evidence of stigmata associated with hyperlipidaemia.
Hazel, a 49-year-old teaching assistant, was referred to the lipid clinic following admission with acute chest pain (subsequently considered non-cardiac, cardiac troponin negative). Her total cholesterol at the time of admission was 10.0 mmol/L. She had previously undergone thyroidectomy and radio-iodine ablation for papillary thyroid cancer. She had a history of treated hypertension (blood pressure [BP] 126/80 mmHg), was overweight (body mass index [BMI] 30.1 kg/m2), physically active, a moderate drinker (10 units per week), recent ex-smoker and dietary assessment indicated scope for improvement. Both parents had type 2 diabetes and hypercholesterolaemia but no clinical cardiovascular disease. She was discharged on simvastatin 40 mg/day, aspirin 75 mg/day, levothyroxine 125 µg/day and lisinopril 5 mg/day. There was no evidence of stigmata associated with hyperlipidaemia.
At the clinic two months later her total cholesterol was 5.2 mmol/L (low-density lipoprotein [LDL] cholesterol 3.7 mmol/L, triglycerides 1.3 mmol/L, high-density lipoprotein [HDL] cholesterol 1.2 mmol/L) and thyroid profile was now within the target range for suppressive treatment (thyroid stimulating hormone [TSH] <0.05 mU/L, free thyroxine [FT4] 25.2 pmol/L). As post-ablation hypothyroidism was considered a possible cause of her dyslipidaemia on hospital admission, simvastatin was withdrawn to reassess her lipid profile. On repeat lipid testing one month later her total cholesterol was 6.3 mmol/L (LDL cholesterol 4.1 mmol/L, triglycerides 1.5 mmol/L and HDL cholesterol 1.6 mmol/L). Her Framingham 10-year cardiovascular risk was estimated at 13%. Hazel was advised to continue with therapeutic lifestyle interventions and return for repeat lipid testing in six months.
This case scenario highlights the importance of considering secondary causes of dyslipidaemia before instigating lipid-modifying treatment. Hazel’s hypothyroidism due to radio-iodine ablation was the probable cause of dyslipidaemia. Following commencement of levothyroxine her cholesterol returned to more normal levels, and these were maintained after stopping statin therapy.


