The importance of heart rate reduction in chronic stable heart failure (HF) has been highlighted in the recently published Systolic Heart Failure Treatment with If Inhibitor Ivabradine Trial (SHIFT). Patients with an elevated resting heart rate (HR) benefited from additional HR control despite optimal doses of beta blockers. The aim of this study was to define the prescribing patterns of beta blockers and the scope for additional HR control in a ‘real life’ HF population.
We conducted a retrospective analysis of two HF clinics, where patients were referred for protocol-guided, up-titration of HF medications. At each assessment we documented: HR, blood pressure, and HF medications including potential side effects. The primary objective was to identify the proportion of patients who had suboptimal HR control (HR ≥70 bpm) despite optimal conventional HF therapy.
From 172 patient records, 145 (84.3%)could tolerate long-term beta blockade with 57 (33.1%) prescribed the maximum recommended dose. Overall, 101 patients were in sinus rhythm with 31/101 (30.7% having an ejection fraction ≤35% and a resting HR ≥70 bpm.
In conclusion, suboptimal HR control is evident in approximately one in three HF patients in sinus rhythm despite aggressive optimisation of beta blocker therapy. This cohort may benefit from additional HR control.
Introduction
Beta-adrenoceptor blocking drugs (beta blockers) are an established prognostic therapy for chronic heart failure (HF).1-4 Of the many proposed mechanisms mediating these favourable effects, that of heart rate (HR) control is gaining interest.
The Systolic Heart Failure Treatment with Iƒ Inhibitor Ivabradine Trial (SHIFT) reported that ivabradine significantly reduced a combined end point of cardiovascular death or HF hospitalisations in a relatively high-risk HF population with an elevated resting HR.5
HR control, therefore, appears to be both a modifiable risk factor and a disease modifying variable in patients with impaired left ventricular (LV) function and HF. Current strategies to control the sinus rate in HF patients include beta blockers, however, up-titration of these drugs can be difficult with patients reporting side effects and/or hypotension.
We, therefore, conducted a retrospective analysis of our HF database to define the prescribing patterns of beta blockers and to identify the proportion of HF patients who may be suitable for additional HR control after optimisation of conventional medical therapy.
Materials and methods
Between April 2007 and September 2010, data were collected from 172 patients referred to two nurse facilitated, specialist HF clinics. Patients were referred to these services for initiation and/or optimisation of medical therapy from secondary care physicians and cardiologists after establishing a diagnosis of HF.
The data collected at each visit included: New York Heart Association (NHYA) class; cardiac medications; resting HR: radial pulse rate over one minute; blood pressure (BP); weight and electrocardiogram (ECG; if HR <50 bpm). Optimisation visits were scheduled bimonthly with slow up-titration of medical therapy based on National Institute for Health and Clinical Excellence (NICE) guidelines,6 with careful monitoring of side effects, HR, BP and biochemistry. We aimed to establish all patients on both angiotensin-converting enzyme (ACE) inhibitors and beta blockers.
Optimisation of medications was frequently assessed at a multi-disciplinary team (MDT) meeting. Titration continued until either the patient was taking the maximum recommended dose of a medication or reported side effects. The titration end points were a systolic BP <100 mmHg with symptomatic hypotension, resting HR <50 bpm or side effects including exacerbations of airways disease, psoriasis or intractable lethargy. Side effects attributed to the beta blocker were addressed by either reducing the dose of beta blocker or discontinuing other antihypertensive medications.7 Up-titration was terminated only after MDT agreement that a patient was prescribed the optimal dose of HF medication
Continuous variables were expressed as mean ± standard deviation (SD) and paired data were analysed by a two-tailed Student’s t-test, p<0.05 was considered significant.
Results
From the 172 referrals, 125 (72.6%) were for up-titration of existing therapy, and 47 (27.3%) for initiation of beta blockers. The baseline demographics are illustrated in table 1.

The patients referred for initiation of beta blocker therapy included subjects that had previously failed initiation of beta blockers. In this challenging cohort of patients, we were able to successfully initiate beta blockers in 27/47 patients, while 20 patients were intolerant. Intolerance to beta blocker initiation was only accepted after failing the protocol-guided system and after discussion with the MDT. Causes for beta blocker intolerances included: severe and limiting hypotension 48%; intractable lethargy 26%; and hospitalisation with severe reversible airways disease 26%. Seven subjects initially on a beta blocker were unable to tolerate long-term therapy.
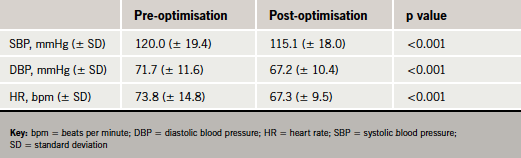
Overall, 145/172 (84.3%) patients could tolerate beta blocker therapy and 27 (15.7%) patients could not tolerate beta blockers. In total, 57/172 (33.1%) subjects could tolerate maximum dose and 110/172 (65.0%) could tolerate ≥50% maximum recommended doses of beta blockers. The haemodynamic profiles before and after optimisation are illustrated in table 2.
Of the 172 patients, 72 (41.9%) had a resting heart rate ≥70 bpm. From this group of patients, 26/72 (36.1%) achieved their target dose of beta blockers, 36/72 (50%) ≥50% of maximum dose of beta blocker and 17/72 (23.6%) were unable to tolerate beta blockers After filtering the data, 31 (30.7%) patients in sinus rhythm, ejection fraction ≤35% had suboptimal HR control (figure 1).

Discussion
HR control remains suboptimal (HR ≥70 bpm) in 41.9% of patients after optimisation of conventional medical therapy despite 84.3% of patients receiving beta blockers and half of this cohort taking at least 50% of the maximum recommended dose. The side effect profile and hypotensive effects of beta blockers limit their up-titration and scope to effectively control HR. However, we were able to establish 27/47 (57.4%) of patients on beta blockers who had previously discontinued this therapy.
The primary site of inhibition of the Iƒ ionic current is the sinus node. Therefore, the 18 subjects in atrial fibrillation with ejection fraction ≤35% and HR ≥70 bpm would not benefit from an Iƒ inhibitor despite suboptimal HR control. This reflects the high burden of atrial fibrillation (41.3%) in our HF population.
The subgroup of patients most likely to benefit from additional HR control are the subjects who are either intolerant or who take ≤50% of maximum dose of beta blocker. In our study there were 11/31 (35.5%) subjects in sinus rhythm, ejection fraction ≤35% and HR ≥70 bpm taking either ≤50% maximum dose of beta blocker or no beta blocker.
This study highlights the prescribing habits and haemodynamic profiles of patients attending an established HF clinic. The limitations of this study include the retrospective data acquisition and the sample size. However, the data do reflect ‘real life’ practice as compared with data from randomised-controlled trials.
Conclusion
The majority of patients with chronic stable HF are able to tolerate beta blockers, but HR control is suboptimal in under half of cases. Close scrutiny of patients in established HF clinics will identify patients that may attain further clinical outcome benefits from newer HF agents, even in the face of aggressive uptitration of current drug regimens.
Conflict of interest
SJR is supported by an educational grant from St Jude Medical. MO, LE, JD, HR, HL-G, VS, AR, RA, ZRY: none declared.
Key message
- Despite the widespread use of beta blockers in heart failure, approximately one in three patients in sinus rhythm with severely impaired left ventricular function have suboptimal heart rate control (resting heart rate >70 bpm)
References
- Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis Ann Intern Med 2001;134:550–60.
- Hjalmarson A, Goldstein S, Fagerberg B et al. Effects of controlled release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA 2000;283:1295–302.
http://dx.doi.org/10.1001/jama.283.10.1295 - Packer M, Fowler MB, Roecker EB et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002;106:2194–9. http://dx.doi.org/10.1161/01.CIR.0000035653.72855.BF
- Anonymous. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial.. Lancet 1999;353:9–13. http://dx.doi.org/10.1016/S0140-6736(98)11181-9
- Swedberg K, Komajda M, Bohm M et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875–85. http://dx.doi.org/10.1016/S0140-6736(10)61198-1
- National Institute for Health and Clinical Excellence. CG108. Chronic heart failure. London: NICE, 2003.
- McMurray J, Cohen-Solal A, Dietz R et al. Practical recommendations for the use of ACE inhibitors, beta-blockers and spironolactone in heart failure: putting guidelines into practice. Eur J Heart Fail 2001;3:495–502. http://dx.doi.org/10.1016/S1388-9842(01)00173-8
