Despite statin use to lower low-density lipoprotein cholesterol, a residual cardiovascular risk remains in dyslipidaemic patients, particularly when high-density lipoprotein (HDL) levels are low. Increased cholesteryl ester transfer protein (CETP) activity is a major determinant of low HDL-cholesterol. CETP inhibition with anacetrapib, evacetrapib and dalcetrapib produces plasma HDL increases of approximately 140%, 80% and 30%, respectively, in patients already receiving statin therapy. However, recent research challenges whether raising HDL-cholesterol is in itself beneficial unless anti-atherogenic properties of HDL, such as cholesterol removal from arterial walls, stimulation of endothelial nitric oxide production or protection against oxidation and inflammation, are enhanced. Potentially important differences are emerging in the mechanisms by which CETP inhibitors operate, which may lead to variation in their anti-atherogenicity unrelated to the changes in HDL-cholesterol they induce. The outcome of clinical trials with CETP inhibitors may thus depend on the mechanisms by which they inhibit CETP. This review discusses clinical implications of CETP inhibition.
Introduction
Statins have proved beyond doubt the relevance of reducing low-density lipoprotein (LDL) in atherogenic cardiovascular disease (CVD), with the risk of a new CVD event reducing by one-fifth for each 1 mmol/L decrease in LDL-cholesterol (LDL-C) achieved.1 A typical patient experiencing an acute coronary event in the UK will do so with LDL-C of 3.8 mmol/L.2 Thus, introducing a statin to achieve a target of just under 2 mmol/L will decrease the risk of a future event by no more than 40%. Unlike more uncommon patients with much higher LDL-C levels, such as those with heterozygous familial hypercholesterolaemia,3 the pre-treatment LDL-C levels of typical patients are simply not high enough, even with an aggressive therapeutic LDL-C target of 2 mmol/L, to benefit from the 50–60% decrease in LDL-C that statins can produce,4 or the even greater LDL-C reduction that can be realised by combining them with newer LDL-C lowering agents, such as ezetimibe5 or colesevelam.6 Therefore, for many patients, a substantial risk persists after LDL-C lowering treatment has been introduced.
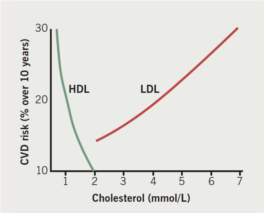
Attention is, therefore, drawn to the prevalence of low plasma high-density lipoprotein (HDL) cholesterol in CVD.7 There is an inverse relationship between HDL-cholesterol (HDL-C) and CVD incidence (figure 1),8 leading to the hypothesis that therapies aimed at raising HDL-C might ameliorate future CVD risk. It is important to recognise that anomalies exist in this relationship (discussed later), which have relevance to which aspects of HDL metabolism to target with pharmacotherapy. Currently prescribed lipid-modifying drugs are disappointing with regard to HDL-C-raising. Statins and fibrates do so usually by no more than 10%, and ezetimibe and bile acid sequestrating agents by even less.3 Of the statins, rosuvastatin is the most potent at raising HDL-C, and, of the fibrates, gemfibrozil is the most effective, but it is also the least suitable fibrate to combine with statin treatment because it also interferes with hepatic elimination of statins by glucuronidation, increasing the likelihood of myositis unacceptably. Although nicotinic acid can increase HDL-C, doubts remain about its tolerability even in combination with laropiprant to decrease the severity of flushing reactions.9 Cholesteryl ester transfer protein (CETP) inhibition, however, offers the prospect of doubling HDL-C levels.10-13
Potential anti-atherogenic activities of HDL
In the adult human, for reasons which have never been adequately explained, the export of cholesterol into the circulation from the liver and gut grossly exceeds the quantity consumed by the rest of the body (largely through the skin as sebum, converted to steroid hormones and vitamin D or used for tissue repair).14 For excess cholesterol entering the circulation, the liver is the major route of elimination. The passage of cholesterol to the liver is termed reverse cholesterol transport (RCT) and, in a state of homeostasis, it balances that which enters the circulation and is not consumed peripherally. It has long been assumed that the main reason HDL protects against atherosclerosis is because atherosclerosis represents the excess deposition of cholesterol in the arterial wall and HDL has a critical role in RCT.15 This is called into question by recent evidence.
First, it is now recognised that the cholesterol in HDL is derived directly from the liver, as well as being incorporated into it in the course of RCT: a significant proportion of cholesterol entering the circulation from hepatocytes does so, not in very low-density lipoproteins, but directly through a transporter channel, the adenosine triphosphate (ATP) binding cassette A1 (ABCA1), and, once in the circulation, it is taken up by small newly secreted HDL particles (figure 2).16 This is a major source of HDL-C. In analphalipoproteinaemia (Tangier disease), for example, in which ABCA1 is mutated, HDL-C levels are virtually non-existent because the small newly secreted HDL particles cannot receive sufficient cholesterol to grow to the point where they are large enough to avoid filtration through the renal glomerulus and subsequent renal catabolism.17
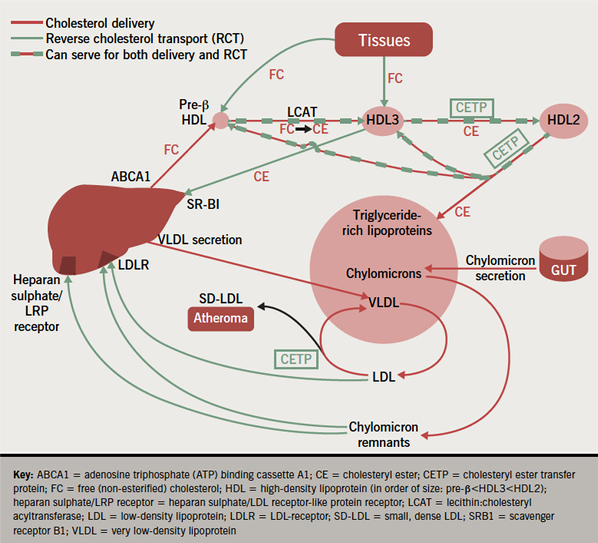
Second, that HDL is essential for RCT is by no means proven and recent evidence has provided alternative mechanisms for the anti-atherosclerotic activity of HDL. Certainly HDL, particularly its smaller and less cholesterol-rich subspecies, pre-β HDL, can stimulate the hydrolysis of intracellular cholesteryl esters and the passage of free cholesterol, thus liberated, across the cell membrane where it can be incorporated into the surface of HDL molecules.7,18,19 The free cholesterol is then re-esterified by lecithin: cholesteryl acyltransferase (LCAT), an enzyme present on HDL. The intensely hydrophobic cholesteryl ester (CE) this produces, is then packed into the core of the HDL away from the surface aqueous interface, creating space at the surface for the addition of more free cholesterol and enlarging the HDL molecule as both its core and surface grow. The capacity of HDL to promote cellular cholesterol efflux in an ex vivo model has been reported to correlate more closely with carotid intima-media thickness than HDL-C concentration.20
Once incorporated into HDL particles, CE can also undoubtedly be off-loaded to hepatocytes as the particles pass along the hepatic sinusoids through, for example the scavenger receptor-B1 (SR-B1), thus completing RCT. Infusions of human apolipoprotein A1, (apo A1, the major protein component of HDL), and over expression of several copies of human APOA1 in animal models enhance resistance to experimental atherosclerosis,21 but, perversely, APOA1 ablation does not increase the likelihood of inducing experimental atherosclerosis.22 In humans, intravenous infusion of synthetic apo A1 can cause regression of coronary atheroma,23 but, on the other hand, two inherited disorders that virtually eliminate HDL, analphalipoproteinaemia and LCAT deficiency, are not associated with any marked tendency to atherosclerosis.3,17 Furthermore, in an animal model in which human hepatic ABCA1 was overexpressed, HDL-C levels were increased, but so was susceptibility to atherosclerosis.24 Deletions in the gene cluster that includes APOA1 have been reported to be associated with aggressive atheroma, but these involve another adjacent gene, APOC3.3 All this inconsistency argues that pathways other than the simple uptake of CE directly from HDL by the liver can also perform RCT.
Because of its low aqueous solubility, it was for a long time assumed that CE could not exchange between lipoproteins. There was, however, the puzzling fact that, whereas CE was insoluble in water, it dissolved in plasma.25 The conundrum was explained by the discovery of CETP, which can bind to CE and transport it between lipoproteins.26 Considerable quantities of CE can be transferred from HDL to the triglyceride-rich lipoproteins, very low-density lipoprotein (VLDL) and chylomicrons, which for physico-chemical reasons are particularly avid acceptors of CE. VLDL and chylomicrons are converted to LDL and chylomicron remnants, respectively, in the circulation. CE is cleared from the circulation when these are taken up by the liver via receptors, such as the LDL-receptor and the heparan sulphate/LDL receptor-like protein receptor (heparan sulphate/LRP receptor).16,27 Thus, LDL and chylomicron remnants, conventionally regarded as unequivocally pro-atherogenic, can in fact contribute to RCT. Furthermore, it was found that cholesterol was released from a variety of tissues complexed to apolipoprotein E, which is also taken up by the liver by both its LDL-receptors and the heparan sulphate/LRP receptor.28
Therefore, while experimentally raising circulating HDL-C to unphysiologically high levels can lead to atheroma regression and this needs to be explored as potential therapy, RCT cannot be the explanation for the strong inverse relationship between HDL-C and CVD risk in the general population in which relatively small differences in HDL-C concentration correlate with substantial variation in CVD incidence compared even with LDL-C (figure 1).
Anti-atherosclerotic activity of HDL not involving RCT
In recent years, increasing attention has been paid to potentially anti-atherogenic components of HDL that may modulate inflammation or protect other lipoproteins, such as LDL, and tissue proteins against atherogenic modification by oxidation or glycation.16,18 Simply raising HDL-C levels without favourably changing these aspects of its function may not realise therapeutic dividends and could explain some of the anomalies in the relationship between HDL-C and CVD, for example the marked effect of insulin in treated type 1 diabetes, which frequently causes high HDL-C levels despite an astronomic CVD risk.3
Despite evidence that cholesterol-laden arterial wall foam cells derived from monocyte-macrophages and LDL are fundamental to the development of atheroma, a dilemma persists because LDL uptake by macrophages is too slow to permit foam cell formation. The prevailing theory to explain this is that the principal protein component of LDL, apolipoprotein B (apo B), must undergo a chemical modification so that it becomes a ligand with high affinity for macrophage scavenger receptors. The most likely modifications of apo B are by lipid peroxidation products18,19 or by glycation.29 HDL has been shown to protect against the accumulation of lipid peroxides on LDL. Several HDL components have been implicated in this, perhaps the most likely being its major protein component, apo A1,18,19 or paraoxonase 1.30,31 Inflammatory mediators are also important in atherogenesis, probably through mediating the recruitment of monocytes and lymphocytes into the developing lesion and the proliferation and transformation of arterial wall smooth muscle cells to acquire fibroblast characteristics. Experimental evidence, largely derived from cultured endothelial cells, suggests that HDL can inhibit vascular cell adhesion molecule 1 (VCAM-1), inter-cellular adhesion molecule (ICAM-1), and endothelial-leukocyte adhesion molecule 1 (E-selectin) expression.18 Furthermore, HDL has recently been reported to increase endothelial nitric oxide production, a capacity that is defective in coronary heart disease (CHD) patients.32
HDL and CETP
Since the discovery of CETP over 20 years ago,26,27 it has been intriguing how often increased CETP activity is associated with conditions linked with accelerated atherosclerosis including diabetes, metabolic syndrome and the dyslipidaemia typically found in myocardial infarction survivors.33 CETP, a hydrophobic glycoprotein, is a member of a family of proteins expressed in species including man and rabbit, which are susceptible to atherosclerosis, but not in rats, which are resistant.
CETP reduces circulating HDL-C levels by transferring esterified CE from HDL to larger lipoproteins, such as chylomicrons, VLDL and LDL, in exchange for triglyceride (figure 2).3 CETP activity is elevated in dyslipidaemia and early onset CHD.33 The literature can be confusing unless it is realised that CETP concentration often correlates poorly with activity measured by the bulk movement of CE through CETP. Indeed, the difficulty of measuring cholesterol flux as opposed to static concentrations bedevils the whole field of RCT. In the case of CETP, it is clear that the concentration of lipoproteins acting as acceptors for CE passing through CETP is often a more important determinant of its activity than its protein concentration.25,34 The larger the lipoproteins and the more triglyceride-rich they are, the more rapid the CETP-mediated transfer of CE to them. This accounts for the increased activity observed in even the most modest hypertriglyceridaemia, something of renewed interest now that triglycerides are established as predictors of CVD.35 This mechanism contributes significantly to lipid homeostasis physiologically too: CETP activity increases postprandially with the influx of larger triglyceride-rich chylomicrons into the circulation.25 Although LDL is a less avid acceptor of CE than VLDL, when its concentrations are particularly high it too can cause raised CETP activity as, for example, in familial hypercholesterolaemia.36
In relation to atherogenesis, CETP activity may be a two-edged sword. It has often been concluded that lowering HDL-C is a bad thing, so, in theory, raising CETP activity should increase atherogenesis. However, in animal models in which CETP is overexpressed, both an increased and a decreased susceptibility to atherosclerosis have been observed depending on the means by which hypercholesterolaemia was induced.26 In the human condition of CETP deficiency due to mutations of CETP, HDL-C concentrations are grossly elevated at 4–5 mmol/L, yet it is unclear whether this confers any substantial protection against CVD.3,26,27 The large, cholesterol-laden HDL particles present in CETP deficiency have reduced capacity to take up cholesterol from macrophages, a potentially important early stage in RCT (see earlier). CETP, by removing CE from HDL, recreates a smaller HDL particle, which is an avid acceptor of cellular cholesterol in experimental models. This has been termed remodelling.18
CETP, by transferring CE from HDL to triglyceride-rich lipoproteins, increases their cholesterol content, which, in turn, increases the cholesterol circulating in the chylomicron remnants and LDL derived from them (figure 2). This would be expected to be a bad thing. However, if hepatic chylomicron remnant and LDL catabolism are not compromised, the liver will receive the CE transferred from HDL and this pathway will, thus, contribute to RCT. In familial hypercholesterolaemia, where LDL catabolism is defective,3 the transfer of additional CE into the circulating LDL pool might, however, be a significant disadvantage.
The exchange of triglyceride and CE between VLDL and LDL promoted by CETP is frequently neglected in discussion of whether it is pro- or anti-atherogenic, but may prove to be even more critical than its effects on HDL. Hypertriglyceridaemia is frequently present in people destined to develop CVD,35 and in those who sustain a coronary event it is associated with a worse prognosis, even after statin treatment.37 CETP activity is almost invariably high in hypertriglyceridaemia, which accounts largely for the low HDL-C accompanying increased plasma triglyceride levels. It also leads to the persistence in the circulation of a small, dense LDL (SD-LDL), which is depleted in cholesterol. Because of this cholesterol depletion, the presence of SD-LDL can only be inferred unless, instead of measuring cholesterol, LDL is quantitated in terms of apo B, its principal protein component, or its particle concentration.38 The generation of SD-LDL under the action of CETP comes about because once CE has been replaced by triglyceride in LDL, the latter is rapidly removed by hepatic lipase to create the small particles that are SD-LDL. SD-LDL is highly atherogenic because it is more susceptible to oxidation and glycation, and may be retained for longer in the arterial subintima than more lipid-rich LDL,18,19,29,39 and is cleared more slowly from the circulation.3,19,29,38
Mechanism of action of CETP inhibitors
Four CETP inhibitors have reached late-stage clinical development: anacetrapib, dalcetrapib, evacetrapib and torcetrapib. Torcetrapib development was halted after increases in cardiovascular events and total mortality were observed in a large outcome trial.40 These adverse effects have been attributed to off-target effects on the renin–angiotensin–aldosterone axis unrelated to CETP inhibition.41 As an add-on to statin therapy, anacetrapib 100 mg daily produced large increases in plasma HDL-C of around 140% with no observable adverse effects on cardiovascular parameters.11,13 Dalcetrapib at a dose of 600 mg daily, also in patients receiving statin treatment, was again without evidence of adverse CVD effects, but the increase in HDL-C of around 30% was, however, more modest.12,42 Perhaps related to this its development was recently halted because in dal-OUTCOMES it lacked efficacy in the secondary prevention of CVD. Interesting differences between dalcetrapib and anacetrapib had, however, by then emerged in terms of their CETP inhibitory mechanism of action.
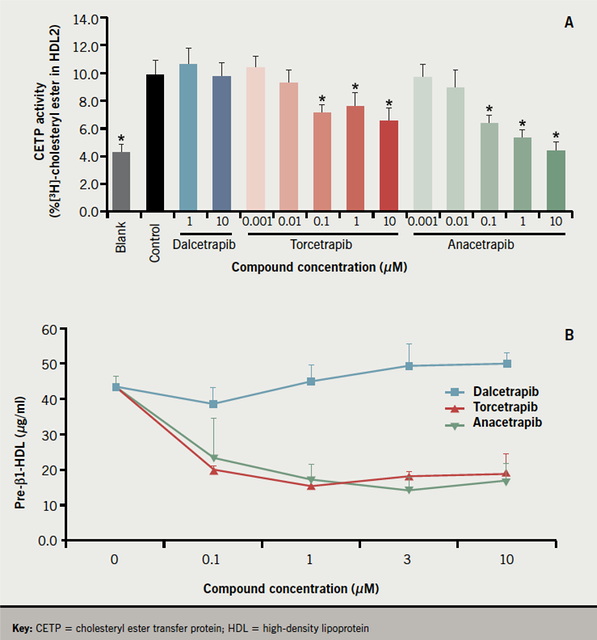
Dalcetrapib binds to a different site on CETP from anacetrapib and uniquely induces a conformational change in the CETP molecule correlating with reduced CETP activity in humans.43 Figure 2 shows the main sites at which CETP blockade can occur: first the transfer of CE from smaller HDL3 particles to HDL2, second the transfer of CE from HDL2 to the triglyceride-rich lipoproteins, thus, regenerating smaller HDL3 and pre-β HDL, and third the transfer of CE from larger LDL particles to triglyceride-rich lipoproteins, creating the highly atherogenic SD-LDL. The effect of CETP inhibitors on each of these processes must be considered. Thus far, more limited data are available about evacetrapib, which in a dose of 100 mg daily in statin-treated patients produces increases in HDL-C of around 80%,44 and pharmacologically, is likely to resemble anacetrapib rather than dalcetrapib.45
Inhibition of transfer of cholesteryl ester from HDL3 to HDL2
The transfer of CE from human HDL3 to HDL2 by recombinant CETP was inhibited by torcetrapib and anacetrapib, but not by dalcetrapib (figure 3A).43
Inhibition of regeneration of HDL3 and pre-β HDL from HDL2
Dalcetrapib had no effect on the generation of pre-β HDL induced in human plasma by recombinant CETP, whereas both torcetrapib and anacetrapib inhibited its formation
(figure 3B).43 On the other hand, all three CETP inhibitors decreased the transfer of CE from HDL to atherogenic LDL. Lipid transfers involving LDL have previously been reported to be more sensitive to partial CETP inhibition by antibodies against CETP than lipid flux through HDL.46
Inhibition of cholesteryl ester transfer out of LDL to create small, dense LDL
So far, results have been published for torcetrapib, where a dose of 120 mg/day decreased the circulating concentration of SD-LDL substantially more than the 17% overall decrease in LDL-C it produced.9 Results with other CETP inhibitors are eagerly awaited because reducing the atherogenicity of LDL may prove to be their most important action.
Effects of CETP inhibition on RCT
Because cholesterol exchanges freely between circulating lipoproteins involved in RCT, it has proved difficult to discover an index of RCT that can be measured in blood samples. However, in hamsters injected with macrophages containing radiolabelled cholesterol, the quantity of radiolabelled cholesterol (neutral sterols) and bile acids (for which cholesterol is a precursor) appearing in the faeces may be regarded as indicative of the removal of excess cholesterol from the body. Dalcetrapib was found to increase radioactive faecal neutral sterols and bile acids and the mass of total faecal bile acids. Despite their much greater effects in raising circulating HDL, lesser effects on faecal neutral sterol and bile acid elimination were observed with torcetrapib, and anacetrapib had no significant activity on these indices of overall RCT.43
Clinical end point trials
A randomised, placebo-controlled two-year study of dalcetrapib in 130 patients, mostly statin-treated, with CHD or at high CHD risk reported no adverse treatment effects, fewer CVD events and some arterial imaging findings, which the authors considered encouraging.47 However, another trial, dal-OUTCOMES, in which it was planned to compare dalcetrapib with placebo in 15,600 patients who had stable CHD following acute coronary syndrome,48 was stopped early because of lack of effect on new CVD events, although there were no safety issues. No doubt the wisdom of designing a trial in which no benefit could be considered to have accrued when only 70% of the events for which it was powered had occurred will be debated widely. However, the decision of the trialists to allow investigators to aim for low LDL targets, if necessary with intensive statin therapy, was correct because any widescale use of CETP inhibitors in the foreseeable future must be against such a statin background because of its established efficacy in preventing CVD.1 Other trials to establish the safety and clinical efficacy in terms of CVD events prevented are currently underway with anacetrapib with its substantially greater effect on HDL (table 1). Evacetrapib is currently in phase II of clinical development.
Conclusions
Details of the steps in lipoprotein metabolism where different CETP inhibitors have their greatest impact are emerging and appear to highlight divergent effects on HDL particles. These may be important clinically because some modulations of HDL metabolism, even though they raise circulating HDL levels, may be less anti-atherogenic than others. Future studies should examine this further. Genetic CETP deficiency results in abnormally large, cholesterol-saturated HDL particles, which are poor cholesterol acceptors.16,26 It may be sufficient to achieve partial CETP inhibition that results in increased smaller HDL particles that are acceptors of cholesterol from arterial wall macrophages, such as pre-β HDL, and decreased formation of atherogenic SD-LDL, while not causing CE to accumulate on large HDL, may be desirable.
We know that overexpression of hepatic scavenger receptor class B type 1 (SR-B1) reduces atherosclerosis in animal models despite the seeming paradox of also reducing plasma HDL levels – supporting the premise that cholesterol throughput is more important than HDL concentration.49 The HDL-raising effect of oestrogens, which did not translate into clinical benefit when subjected to randomised clinical trials, may be, in part, due to down-regulation of SR-B1.50 Furthermore, trials with torcetrapib showed no consistent relationship between plasma HDL levels and atherosclerosis despite marked increases in plasma HDL of around 60%.51
Clearly, the relationship between plasma HDL and atherosclerosis is a more complex one than merely ‘high levels are good’. However, the recent failure of dalcetrapib to ameliorate CVD in dal-OUTCOMES may have stemmed from its modest effect in raising HDL compared with anacetrapib and evacetrapib. None the less, the work undertaken during its development emphasises that, in harnessing the therapeutic potential of agents that modify HDL metabolism, attention must be paid to its heterogeneity. The anticipated benefits from raising its concentration may not be realised unless its function is also favourably modified.
Acknowledgements
I am grateful to Katherine Mantell of Virgo HEALTH Education for assistance with the literature review and for expert preparation of this manuscript. This aspect of the work was funded by an unrestricted grant from Hoffmann-La Roche. The opinions expressed here are entirely those of PND and have not been reviewed by anyone else.
Conflict of interest
None declared.
Editors’ note
See also the editorial on this article by Jonathan Morell on pages 104–106 of this issue.
Key messages
- Raising circulating HDL can protect against atherosclerosis, but the mechanism by which it is accomplished may be critical
- CETP inhibition is a potent means of increasing HDL
- By blocking transfer of cholesteryl ester from HDL to other lipoproteins, CETP inhibition may promote uptake by the liver and other anti-atherogenic properties of HDL, but it might also impair regeneration of small HDL particles necessary for removal of excess tissue cholesterol
- Novel drugs varying in the extent to which they inhibit CETP are currently under development
References
- Baigent C, Blackwell L, Emberson J et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. http://dx.doi.org/10.1016/S0140-6736(10)61350-5
- Chaudhury M. Blood analytes. In: Sproston K, Primatesta P, eds. Health Survey for England 2003: risk factors for cardiovascular disease. London: The Stationery Office, 2004:241–87.
- Durrington PN. Hyperlipidaemia diagnosis and management. 3rd ed. London: Hodder Arnold, 2008.
- Soran H, Durrington P. Rosuvastatin: efficacy, safety and clinical effectiveness. Expert Opin Pharmacother 2008;9:2145–60. http://dx.doi.org/10.1517/14656566.9.12.2145
- Ara R, Pandor A, Tumur I et al. Estimating the health benefits and costs associated with ezetimibe coadministered with statin therapy compared with higher dose statin monotherapy in patients with established cardiovascular disease: results of a Markov model for UK costs using data registries. Clin Ther 2008;30:1508–23. http://dx.doi.org/10.1016/j.clinthera.2008.08.002
- Huijgen R, Abbink EJ, Bruckert E et al. Colesevelam added to combination therapy with a statin and ezetimibe in patients with familial hypercholesterolemia: a 12-week, multicenter, randomized, double-blind, controlled trial. Clin Ther 2010;32:615–25. http://dx.doi.org/10.1016/j.clinthera.2010.04.014
- Rader DJ, Alexander ET, Weibel GL et al. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res 2009;50(suppl):S189–S194. http://dx.doi.org/10.1194/jlr.R800088-JLR200
- Anderson KM, Wilson PW, Odell PM et al. An updated coronary risk profile. A statement for health professionals. Circulation 1991;83:356–62.
- Lai E, De Lepeleire I, Crumley TM et al. Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype 1. Clin Pharmacol Ther 2007;81:849–57. http://dx.doi.org/10.1038/sj.clpt.6100180
- Brousseau ME, Schaefer EJ, Wolfe ML et al. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N Engl J Med 2004;350:1505–15. http://dx.doi.org/10.1056/NEJMoa031766
- Krishna R, Anderson MS, Bergman AJ et al. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomised placebo-controlled phase I studies. Lancet 2007;370:1907–14. http://dx.doi.org/10.1016/S0140-6736(07)61813-3
- Stein EA, Roth EM, Rhyne JM et al. Safety and tolerability of dalcetrapib (RO4607381/JTT-705): results from a 48-week trial. Eur Heart J 2010;31:480–8. http://dx.doi.org/10.1093/eurheartj/ehp601
- Cannon CP, Shah S, Dansky HM et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med 2010;363:2406–15. http://dx.doi.org/10.1056/NEJMoa1009744
- Myant N. The biology of cholesterol and related steroids. London: William Heinemann Medical Books Ltd., 1981.
- Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet 1975;1:16–19. http://dx.doi.org/10.1016/S0140-6736(75)92376-4
- Charlton-Menys V, Durrington PN. Human cholesterol metabolism and therapeutic molecules. Exp Physiol 2008;93:27–42.
- Schaefer EJ, Santos RD, Asztalos BF. Marked HDL deficiency and premature coronary heart disease. Curr Opin Lipidol 2010;21:289–97. http://dx.doi.org/10.1097/MOL.0b013e32833c1ef6
- Tabet F, Rye KA. High-density lipoproteins, inflammation and oxidative stress. Clin Sci (Lond) 2009;116:87–98. http://dx.doi.org/10.1042/CS20080106
- Chapman MJ, Le GW, Guerin M et al. Cholesteryl ester transfer protein: at the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur Heart J 2010;31:149–64. http://dx.doi.org/10.1093/eurheartj/ehp399
- Khera AV, Cuchel M, Llera-Moya M et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127–35. http://dx.doi.org/10.1056/NEJMoa1001689
- Duverger N, Kruth H, Emmanuel F et al. Inhibition of atherosclerosis development in cholesterol-fed human apolipoprotein A-I-transgenic rabbits. Circulation 1996;94:713–17.
- Plump AS, Azrolan N, Odaka H et al. ApoA-I knockout mice: characterization of HDL metabolism in homozygotes and identification of a post-RNA mechanism of apoA-I up-regulation in heterozygotes. J Lipid Res 1997;38:1033–47.
- Nicholls SJ. High-density lipoprotein and progression rate of atherosclerosis in intravascular ultrasound trials. Am J Cardiol 2009;104:16E–21E. http://dx.doi.org/10.1016/j.amjcard.2009.09.015
- Feng Y, Lievens J, Jacobs F et al. Hepatocyte-specific ABCA1 transfer increases HDL cholesterol but impairs HDL function and accelerates atherosclerosis. Cardiovasc Res 2010;88:376–85. http://dx.doi.org/10.1093/cvr/cvq204
- Channon KM, Clegg RJ, Bhatnagar D et al. Investigation of lipid transfer in human serum leading to the development of an isotopic method for the determination of endogenous cholesterol esterification and transfer. Atherosclerosis 1990;80:217–26. http://dx.doi.org/10.1016/0021-9150(90)90029-I
- Masson D, Jiang XC, Lagrost L et al. The role of plasma lipid transfer proteins in lipoprotein metabolism and atherogenesis. J Lipid Res 2009;50(suppl):S201–S206. http://dx.doi.org/10.1194/jlr.R800061-JLR200
- Barter PJ, Brewer HB Jr., Chapman MJ et al. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol 2003;23:160–7. http://dx.doi.org/10.1161/01.ATV.0000054658.91146.64
- Langer C, Huang Y, Cullen P et al. Endogenous apolipoprotein E modulates cholesterol efflux and cholesteryl ester hydrolysis mediated by high-density lipoprotein-3 and lipid-free apolipoproteins in mouse peritoneal macrophages. J Mol Med 2000;78:217–27. http://dx.doi.org/10.1111/j.1755-5922.2010.00142.x
