The very public resuscitation of a premier league footballer drew nationwide attention to fatal dysrrhythmias in the young. Survival was achieved by effective bystander cardiopulmonary resuscitation (CPR), rapid transportation and targeted resuscitation in a cardiac centre. In Emergency Departments in the UK, resuscitation from shockable dysrrhythmias follows the Advanced Life Support (ALS) protocol, using biphasic defibrillation (150–200 J) with subsequent adrenaline boluses and amiodarone. In patients with hypertrophic cardiomyopathy or primary ventricular arrhythmias without structural heart disease, high energy defibrillation (up to 360 J) is sometimes required and catecholamines predispose to recurrent dysrrhythmia.1 On occasion, more can be learnt from failure than success. For this reason we present the following case.
Case report
A 20-year-old female student under investigation for syncopal attacks was found to have a normal electrocardiogram (ECG) and cardiac morphology on echocardiography. She then suffered ventricular fibrillation at rest while talking to friends. They performed cardiac massage and a paramedic ambulance arrived within four minutes. Defibrillation was attempted using anterior and lateral electrodes. When this was unsuccessful, she was intubated and a Lucas cardiac compression device applied, even though the Accident and Emergency (A&E) department was less than one mile away. When she arrived at the hospital 30 minutes after presentation, the dysrrhythmia again proved resistant to defibrillation at 150 J. Following the ALS resuscitation protocol, adrenaline boluses and amiodarone were given. After more than 30 shocks the on-call cardiologist (an electrophysiologist) arrived and changed the defibrillator electrodes to the antero-posterior position. With a shock voltage of 200 J, sinus rhythm was restored providing a blood pressure of 150/100 mmHg. With high circulating catecholamine levels, ventricular fibrillation recurred repeatedly, but stable sinus rhythm was eventually established (after 80 minutes) using high-dose infusions of lignocaine and the beta blocker esmolol. Following review by a consultant intensivist and cardiac surgeon she was transferred to the cardiac intensive care unit.
Over the following six hours, the blood pressure fell to 80/60 mmHg with central venous pressure of 20 mmHg. She became acidotic and oliguric. Transoesophageal echocardiography showed severe biventricular dysfunction, secondary to prolonged cardiac massage, more than 70 cardioversions and high-dose antidysrrhythmic therapy. An intra-aortic balloon pump (IABP) was inserted but re-introduction of noradrenaline caused further episodes of ventricular fibrillation. The low cardiac output state was not significantly improved by the IABP and she remained oliguric and acidotic. At the time, her pupils remained reactive to light but it became clear that the only route to survival was mechanical circulatory support. With worsening blood gases and raised venous pressure, veno-arterial extracorporeal membrane oxygenation (ECMO) was the preferred method. We would have liked to have used levosimendan, which sensitises cardiac troponin C to the effects of intra-cellular calcium.2 This improves myocardial contractility without increasing ventricular irritability or oxygen consumption. It also lowers pulmonary vascular resistance and increases cardiac output. Unfortunately, it was not available. Coronary angiography with low-contrast dose was performed to rule out a coronary anomaly. This proved normal.
The decision to employ ECMO was made at 6.30 am and contact was made with a very responsive device manufacturer by 8.30 am. The equipment was delivered at around 1.00 pm. During the intervening period, systolic blood pressure fell to 60 mmHg, while the central venous pressure rose to 25 mmHg. This severely compromised the cerebral perfusion gradient.
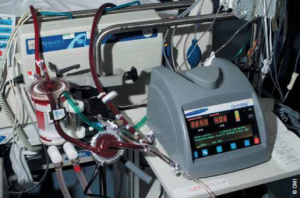
It took only a few minutes to establish ECMO (figure 1). Ultrasound of the femoral vessels showed the right femoral artery to be small, secondary to high bifurcation under the inguinal ligament. Because of this, the artery was exposed and a 6 mm vascular graft anastomosed in order to prevent distal limb ischaemia by direct cannulation. The left femoral vein was cannulated by the Seldinger technique, and a long venous cannula introduced into the right atrium guided by transoesophageal echocardiography. With onset of ECMO support, the systolic blood pressure, still augmented by the IABP, rose rapidly to 110/70 mmHg, with a fall in central venous pressure to between 5 to 8 mmHg. Haemofiltration was then introduced to restore acid–base balance. Urine output improved spontaneously. For the first few hours her pupils remained responsive to light. Then, while left and right ventricular function improved substantially, the pupils became dilated and fixed. A computed tomography (CT) scan was performed to rule out a treatable cerebral haemorrhage. This showed cerebral oedema with herniation of cerebral peduncles through the foramen magnum. When ECMO was discontinued after 48 hours, her haemodynamic status remained good and the kidneys were producing urine. Following formal brain-stem death testing, her family communicated her wish to be an organ donor. She donated liver and kidneys.
Discussion
Idiopathic ventricular fibrillation presents as syncope or sudden cardiac death in young people with normal hearts and no identifiable genetic syndrome.1 The events are typically unrelated to stress or activity but may occur in clusters characterised by frequent ventricular ectopy and short self-terminating episodes of ventricular fibrillation with syncope or even, as in this case, full-blown “electrical storm”.3 Ventricular fibrillation is triggered by premature ventricular complexes which arise from the Purkinje fibres or the myocardium.4 This patient’s electrical storm settled with conservative management but even refractory cases are treatable by electrophysiologic mapping and catheter ablation of the Purkinje network, with excellent long-term outcomes.5
Our patient collapsed in the early evening, less than half a mile from the ambulance station and one mile from a major teaching hospital with regional cardiac surgery and trauma centres. Cardiac massage was performed by her friends but paramedics were unable to achieve defibrillation using antero-lateral electrodes. In retrospect, we wonder whether application of the Lucas cardiac compression system delayed transfer to the A&E department.
Once in the Emergency Department, defibrillation required repositioning of the electrodes and an increase in energy to 200 J. High-dose beta-blockade was then needed to counter the effects of catecholamines. After two hours of cardiac massage, electrical stunning and beta-blockade, biventricular failure was inevitable. Though widely available, the IABP does not significantly increase cardiac output (<500 ml/min) and numerous clinical trials have failed to demonstrate survival benefit in established cardiogenic shock.6
For patients who are already on an IABP, a serum lactate >11 mmol/L, base deficit >12 mmol/L, mean arterial pressure <55 mmHg and oliguria (<50 ml over two hours) herald impending death without mechanical circulatory support.6 For acute biventricular failure, ECMO can be instituted rapidly to ensure adequate systemic blood flow and unload the failing right ventricle.7 Elevated systolic pressure with reduced central venous pressure then improves tissue perfusion pressure, and oxygen delivery. Either ECMO or temporary centrifugal blood pump support without an oxygenator can salvage between 50 to 70% of cardiogenic shock patients depending on aetiology.8
The European guidelines for myocardial revascularisation recommend ECMO as first-line management for cardiogenic shock.9 European and North American mainstream cardiac intervention centres employ ECMO routinely. Helicopter- or ambulance-based portable devices are used for rescue then transport from district to tertiary care hospitals.10 ECMO circuits are deployed in minutes using percutaneously inserted cannulas even during cardiac massage. For outreach retrieval the district cardiologist can introduce femoral arterial and venous guide wires before the transport team arrives.10 In the UK, ECMO has been made available only in a small number of centres involved in cardiopulmonary transplantation and respiratory support, the majority of which do not even have an Emergency Department.
We obtained ECMO for this patient and achieved both cardiac and end-organ recovery. The single most important factor was the six-hour delay between establishing clinical need and delivery of an ECMO system to the hospital. The critical balance between low mean perfusion pressure versus elevated central venous pressure and worsening cerebral oedema caused irreversible brain injury. Crucially, neither levosimendan nor the ECMO circuit were available to the clinical team.2 We have previously highlighted the restrictions on life-saving technology in UK cardiac centres.11 The National Specialist Commissioning Group’s standpoint that only a small number of cardiac centres can manage ECMO, contravenes National Institute for Health and Clinical Excellence (NICE) guidelines for temporary circulatory support (2006), European Society of Cardiology guidelines on myocardial revascularisation (2011), and General Medical Council guidelines on end-of-life care (2011).9,12,13
It is also noteworthy that, had this patient’s electrical storm not been controlled medically (which was a distinct possibility at presentation), effective catheter ablation probably would not have been feasible without the circulatory support provided by ECMO.14
Despite the recent case in the media (which did not follow a conventional National Health Service [NHS] route to an Emergency Department), the NHS is not equipped to salvage such patients, given restrictions on medicines and technology. The current ‘Safe and Sustainable’ strategy for congenital heart surgery will further reduce the number of centres able to intervene in young people who present acutely with immediately life-threatening cardiac problems. Many of these patients are not suitable for inter-hospital transfer, even if the NHS had a comprehensive helicopter-based transfer system to allow this to happen.
Were we correct to demand ECMO for this young patient who could not be transferred? First, the consultant-based medical team had extensive experience of mechanical circulatory support including ECMO. Second, Article 2 of the European Convention on Human Rights defines “the right to life and a positive duty on medical staff and organisations to preserve life and palliate distressing symptoms”. The General Medical Council’s guidelines for end-of-life care state that “you should not withhold treatment if doing so would involve significant risk for the patient and the only justification is resource constraints” (Point 39, page 27).13 The presumption exists that all reasonable steps will be taken to prolong life if the treatment concerned is based on contemporary evidence. Failure to treat a potentially recoverable young patient is frankly negligent. Thus, all tertiary cardiac centres must have the capacity to deal with cardiogenic shock and have access to the necessary equipment.
In this case, restrictions in availability of appropriate equipment precluded a satisfactory outcome through cerebral injury. Other organs were preserved and used for transplantation. Will lessons be learnt from this tragic loss?
Conflict of interest
None declared.
Editors’ note
An editorial on this topic by Sharma et al. can be found on pages 102–03 of this issue.
References
- Prystowsky EN, Padanilam BJ, Joshi S, Fogel RI. Ventricular arrhythmias in the absence of structural heart disease. J Am Coll Cardiol 2012;59:1733–44. http://dx.doi.org/10.1016/j.jacc.2012.01.036
- Kolseth SM, Wahba A, Kirkeby-Garstad I et al. A dose-response study of levosimendan in a porcine model of acute ischemic heart failure. Eur J Cardiothorac Surg 2012;41:1377–83. http://dx.doi.org/10.1093/ejcts/ezr201
- Haissaguerre M, Derval N, Sacher F et al. Sudden cardiac arrest associated with early repolarisation. N Engl J Med 2008;358:2016–23. http://dx.doi.org/10.1056/NEJMoa071968
- Haissaguerre M, Stroda M, Jais P et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation 2002;106:962–7. http://dx.doi.org/10.1161/01.CIR.0000027564.55739.B1
- Knecht S, Sacher F, Wright N et al. Long term follow up of idiopathic ventricular fibrillation ablation. J Am Coll Cardiol 2009;54:522–8. http://dx.doi.org/10.1016/j.jacc.2009.03.065
- Westaby S, Anastasiadis K, Weiselthaler GM. Cardiogenic shock in ACS. Part 2: Role of mechanical circulatory support. Nat Rev Cardiol 2011;9:195–208. http://dx.doi.org/10.1038/nrcardio.2011.205
- Formica F, Avalli L, Redoelli G, Paolini G. Interhospital stabilisation of adult patients with refractory cardiogenic shock by veno-arterial extracorporeal membrane oxygenation. Int J Cardiol 2011;147:164–5. http://dx.doi.org/10.1016/j.ijcard.2010.09.062
- Westaby S, Kharbanda R, Banning AP. Cardiogenic shock in ACS. Part I: prediction, presentation and medical management. Nat Rev Cardiol 2012;9:158–71. http://dx.doi.org/10.1038/nrcardio.2011.194
- Joint Task Force on Myocardial Revascularisation of the European Society of Cardiology and European Association of Cardiothoracic Surgery. Guidelines on Myocardial Revascularisation. Eur Heart J 2010;31:2501–56.
- El-Banayosy A, Cobaugh D, Zitterman A et al. A multidisciplinary network to save the lives of severe persistent cardiogenic shock patients. Ann Thorac Surg 2005;80:543–7. http://dx.doi.org/10.1016/j.athoracsur.2005.03.039
- Westaby S, Taggart D. Inappropriate restrictions on life saving technology. Heart 2012;98:1117–19.
- National Institute for Health and Clinical Excellence. Interventional Procedure Guidance 177. Short term circulatory support with left ventricular assist devices as a bridge to cardiac transplantation or recovery. London: NICE, June 2006.
- General Medical Council. Treatment and care towards the end of life: good practice in decision making. London: GMC, 20 May 2010. Available from: http://www.gmc-uk.org/guidance/ethical_guidance/end_of_life_care.asp [accessed 11 January 2012].
- Carbucicchio C, Della Bella P, Fassini G, et al. Percutaneous cardiopulmonary support for catheter ablation of unstable ventricular arrhythmias in high-risk patients. Herz 2009;34:545–52.
