There is a lack of clarity around the current use of anti-arrhythmic drugs (AADs), highlighted by the recent changes to the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) recommendations for dronedarone use, which were in response to the early termination of the Permanent Atrial Fibrillation Outcome Study Using Dronedarone On Top Of Standard Therapy (PALLAS) trial due to increased mortality in the dronedarone arm. A UK- and Ireland-based multi-disciplinary expert group was convened by Sanofi*, the manufacturers of dronedarone, to address the need for guidance in the practical implementation of guidelines for AADs. This position statement provides the group’s evidence-based recommendations for the practical use of AADs and dronedarone in particular. Since AADs are not always used in line with recommendations, the guidelines for the use of AADs, and the evidence base supporting them, are reviewed. The current recommendations for dronedarone use are set within this context. On consideration of the evidence, the recommendation for dronedarone use is for the maintenance of sinus rhythm in non-permanent atrial fibrillation (AF) patients, without severe heart failure, or amiodarone-related liver or lung toxicities, and with appropriate anticoagulation. Given that there have been no new AADs available in 25 years to address the need for an effective anti-arrhythmic with reduced side effects, dronedarone has a place in the treatment of non-permanent AF to provide options for clinicians and patients.
* Further detail of Sanofi’s support is declared in the conflict of interest statement at the end of this article.
Introduction
Patients with atrial fibrillation (AF) can benefit from rhythm management to improve unpleasant symptoms or increase exercise capacity,1 making anti-arrhythmic drugs (AADs) an important option in the management of AF. The benefits of any AAD must be weighed against the risks of adverse effects, which in some cases are serious. Defined indications for the use of AADs have been developed by regulatory bodies such as the European Medicines Agency (EMA) and US Food and Drug Administration (FDA), which, in addition to guidelines from groups such as the UK National Institute for Health and Clinical Excellence (NICE) and the European Society of Cardiology (ESC), enable these drugs to be used safely in the appropriate patients.
Recently, there have been new regulatory approvals given to the AAD dronedarone, which is being used by a growing number of patients worldwide (currently over 600,000). However, the terms of the approvals differ markedly between the FDA and the EMA. Over the last two years, several international guidelines and guideline updates have been put forward by the ESC,1 the Canadian Cardiovascular Society (CCVS)2 and the American College of Cardiology Foundation (ACCF)/American Heart Association (AHA).3 The guidance differs considerably between these documents. NICE issued a Health Technology Appraisal (HTA) approving the use of dronedarone in a limited population of patients with characteristics similar to those recruited in the ATHENA (A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg b.i.d. for the Prevention of Cardiovascular Hospitalisation or Death from Any Cause in Patients with Atrial Fibrillation/Atrial Flutter) trial; guidance which more closely resembled that of the FDA rather than the EMA.4
Throughout the UK and Ireland, formulary committees are struggling to provide local guidance. In order to inform this process, a UK- and Ireland-based expert group with a particular interest in AF was convened consisting of cardiologists, electrophysiologists, hospital physicians, primary care general practitioners and a cardiovascular disease pharmacist. The group met in London on 31 January 2012. The dronedarone evidence base was compared with that of other AADs and combined with field experience from the local experts. EMA and NICE guidance was acknowledged as specifically relevant to UK practice, and advice on the practical interpretation and implementation of their recommendations was assembled. The expert group’s advice is summarised in this document.
Clinical development of dronedarone in AF
For the last 25 years there have been no new AADs available in the UK and Ireland, and the AADs historically used for suppressing AF are of limited efficacy. Their usefulness is further limited by the high incidence of side effects (especially with beta blockers and amiodarone) and risks, particularly of provoking malignant ventricular arrhythmias. No AAD has been shown to reduce mortality in AF populations, and most retrospective studies and meta-analyses suggest that traditional AADs, if anything, increase mortality.5 The alternative for effective rhythm control is catheter ablation, but this is expensive, a major undertaking for the patient, and also carries risks.
The development of dronedarone was underpinned by this need for an effective AAD with fewer side effects and an improved safety profile. Several randomised-controlled trials have demonstrated that dronedarone improves outcomes in AF patients, by maintaining sinus rhythm and, potentially, by lowering risks of cardiovascular (CV) events.6–9 Following encouraging results in a subset of apparently permanent AF patients from the ATHENA study,10 the phase III Permanent Atrial Fibrillation Outcome Study Using Dronedarone On Top Of Standard Therapy (PALLAS) trial was initiated to investigate a potential benefit to patients with permanent AF in a larger study population. However, PALLAS was terminated prematurely due to an increase in mortality in the dronedarone arm at around twice that observed in the placebo arm.11
In response to these events, the EMA and FDA revised the guidance for the use of dronedarone. Despite both regulatory bodies considering the same evidence, the EMA’s response provides more cautious recommendations than that from the FDA, although both have confirmed that dronedarone is still recommended as an active treatment in specified patient groups (table 1). There is a critical need for interpretation of these recommendations to ensure the use of dronedarone in clinical practice is clearly defined in the context of the current use of other AADs.

AADs in clinical practice
All AAD treatments carry an element of risk that must be managed appropriately. Despite a compendium of regulatory recommendations and guidelines, monitoring of AAD treatment is not always carried out appropriately,12 and these drugs are often not used according to regulatory or guideline approval.
The current recommendations for the use of AADs are summarised in tables 2 and 3. In addition, there are particular safety concerns for individual AADs, which should be emphasised.
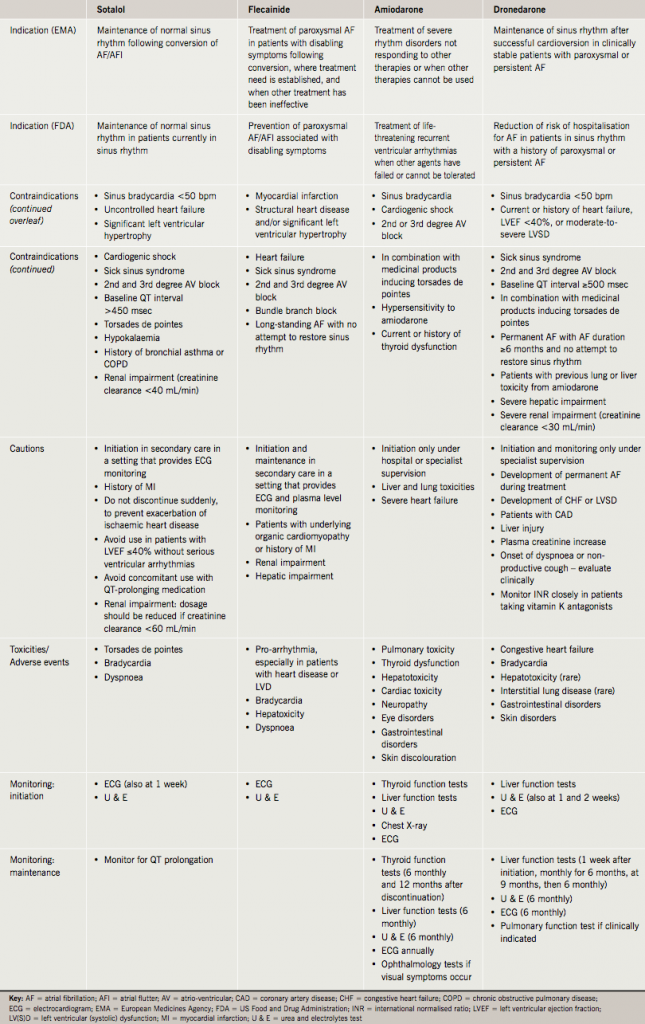

Sotalol
Sotalol is generally available as a racemic mixture (d,l-sotalol). The l-isomer is a simple beta blocker, while the d-isomer also has true anti-arrhythmic action (Vaughan-Williams Class III), which manifests at doses over 80 mg twice daily, and carries the risk of provoking malignant ventricular arrhythmia (torsades de pointes) in susceptible individuals.13 This may be why sotalol is commonly used at doses of 40–80 mg twice daily, at which level it usually has little or no true anti-arrhythmic action. Guidelines recommend an electrocardiogram (ECG) shortly after initiating sotalol and after dose increases because of the risk of torsades de pointes. Dyspnoea is a common side effect (>1% of patients), and sotalol is contraindicated in patients with a history of chronic obstructive pulmonary disease (COPD) or bronchial asthma. It is also contraindicated in patients with significant left ventricular hypertrophy. Potassium and magnesium imbalances should also be corrected before initiation. Sotalol should not be given to patients with renal failure or low body mass index.
Flecainide
The recommendation that flecainide should be initiated in hospital ‘under specialist supervision’ with ECG monitoring is not followed rigorously in practice. Potassium and magnesium imbalances should be corrected before initiation. Flecainide should not be used in patients with congestive heart failure or patients with a history of myocardial infarction because of the increased mortality observed in the Cardiac Arrhythmia Suppression Trial (CAST).14 The deleterious effects of flecainide in CAST have been widely extrapolated to extend its contraindication to patients with coronary artery disease, or any degree of heart failure or left ventricular hypertrophy. Similar considerations apply equally to treatment with propafenone.
Amiodarone
Amiodarone is indicated for the restoration of sinus rhythm when other treatments have failed, because, although it has the greatest efficacy of any AAD at maintaining sinus rhythm, it also has a significant extra-cardiac adverse event profile. It should be used with caution in patients with moderate and severe heart failure as the risk of death was increased in New York Heart Association (NYHA) class III patients treated with amiodarone compared with placebo in the Sudden Cardiac Death in Heart Failure Trial (SCD-HEFT).15 Thyroid toxicity is a common side effect of amiodarone, affecting 3.7–15% of treated patients.16 Abnormal liver and thyroid function tests result in discontinuation in a significant proportion of patients over time. Visual problems, such as corneal microdeposits and optical neuropathy, can occur in 90% and 5% of patients taking amiodarone, respectively, and photosensitivity appears in 25–75% of patients taking amiodarone.17 Serious pulmonary and hepatic toxicity have been reported in association with amiodarone.17
The expert group’s recommendations for the use of dronedarone
Non-permanent AF patients – maintenance of sinus rhythm
Regulatory guidance is supported with respect to dronedarone use for maintaining sinus rhythm in non-permanent AF patients without severe heart failure, with no previous lung and/or liver toxicity from amiodarone, and with appropriate anticoagulation.
Data from the PALLAS study demonstrate that dronedarone should not be used in permanent AF patients due to the increased incidence of stroke and cardiovascular mortality. These hazards appear to be common to all permanent AF patients, as no specific subset of patient characteristics was clearly responsible for the differences in mortality between the dronedarone and placebo arms. The increased risk of stroke and heart failure in the dronedarone arm of the PALLAS study, despite reductions in heart rate and blood pressure (potentially associated with benefit in AF patients with high CV risks), remains unexplained.11 This is at odds with the findings of reduced mortality and cardiovascular hospitalisations in ATHENA. Multiple factors are likely to be involved, especially increased patient age and the burden of cardiovascular disease.11 It has been suggested that additional factors, such as levels of anticoagulation and/or digoxin may also have been relevant, though the importance of these factors remains unclear.
Dronedarone is associated with some risks that are offset by benefits when the drug is helping to maintain sinus rhythm, but not so in permanent AF. Dronedarone should be given, under specialist supervision, to patients either in sinus rhythm or prior to scheduled restoration of sinus rhythm. Preloading with dronedarone prior to cardioversion to increase the likelihood of maintaining sinus rhythm once restored by cardioversion is not inappropriate. To avoid dronedarone being used by patients in permanent AF, dronedarone should be discontinued whenever the patient develops permanent AF. Determining whether a patient is in permanent AF requires regular ECG monitoring (at least every six months, based on the PALLAS trial criteria). Permanent AF is defined when an active management decision has been made to cease attempts to restore sinus rhythm by an appropriately qualified or appropriately trained healthcare professional (HCP).
Considerations for dronedarone use
Heart failure and left ventricular systolic dysfunction
The EMA recommends that dronedarone should not be given to patients with heart failure due to presumed or diagnosed left ventricular dysfunction, as there is evidence of harm from the PALLAS and Anti-arrhythmic Trial with Dronedarone in Moderate to Severe Congestive Heart Failure Evaluating Morbidity Decrease (ANDROMEDA) trials in patients with severe congestive heart failure (NYHA class III–IV). In practice, ‘heart failure or history of heart failure’ should be interpreted as a patient having a documented episode of heart failure (as demonstrated by previous hospitalisation with a diagnosis of heart failure that is not specifically and only due to rapid AF) or an echocardiogram report of a low left ventricular ejection fraction (LVEF) <40%. In these cases, dronedarone should not be prescribed. There is no evidence base for excluding patients with mild left ventricular dysfunction and no symptoms of heart failure. Patients without current heart failure symptoms and only mild left ventricular dysfunction may be eligible for dronedarone, as there was no difference between drug and placebo arms in the PALLAS and ATHENA trials for patients with mild heart failure (NYHA class I–II). Furthermore, patients in whom prior left ventricular dysfunction was a consequence of sustained tachycardia during AF, but subsequently improved, may be eligible for dronedarone. Attention should be paid to the development of congestive heart failure or significant left ventricular systolic dysfunction in routine clinical assessments and dronedarone should be discontinued if these develop.
Coronary artery disease
The EMA recommends caution in patients with coronary artery disease (CAD), although there was little evidence from PALLAS or ANDROMEDA to support this. The FDA did not recommend caution in CAD patients in response to the result of the PALLAS trial. The published evidence base does not provide any reason to show concern in such patients, but the PALLAS trial has yet to be reported in full.
Lung and liver toxicities
Pulmonary toxicities are rare for dronedarone: the pulmonary event rate was 0.6% for dronedarone versus 0.8% for placebo across all five randomised-controlled trials.18 Pulmonary function tests are only required for dronedarone patients when clinically indicated by symptoms such as non-productive cough. If pulmonary toxicity occurs, dronedarone should be discontinued.18
Life-threatening hepatic toxicity is very rare; the incidence rates of hepatocellular liver injury are between one in 1,000 and one in 10,000 for dronedarone,18 compared with <3% incidence of hepatitis and cirrhosis with amiodarone.17 Elevated enzyme levels occur at rates between one in 10 and one in 100 with dronedarone,18 compared with 15–30% with amiodarone.17 Frequent monitoring does not appear to be justified other than in patients who have previously suffered toxicity while on amiodarone. Clinicians should be aware of indications of deteriorating liver function, such as pruritus, in addition to the liver function tests for enzyme levels specified by the EMA, and should withdraw dronedarone until liver function normalises.18 Derangement in international normalised ratio (INR) control should also prompt examination of liver function tests (LFTs), as liver dysfunction can increase the INR.
The EMA currently recommends that dronedarone patients undergo LFTs at initiation, after one week, and at monthly intervals for the first six months, and at nine and 12 months, although it is not known whether such frequent testing can help avoid severe liver damage. The FDA simply advises periodic testing during the first six months of treatment. Use of specialist nurse-led clinics and established protocols can reduce the burden of monthly LFTs. Although there is some justification for frequent LFTs currently, to ensure patient safety and demonstrate the low incidence of serious hepatic adverse events, this requirement should be reviewed in the future. Such a review has recently been made with regard to treatment with statins, for which NICE and the FDA have downgraded the LFT monitoring requirements, as they are no longer considered necessary.
Stroke risk
The incidence of stroke is known to be increased in AF patient populations. Given the increased stroke deaths in the PALLAS trial, the group concurs with EMA and FDA guidance that patients on dronedarone must be appropriately anticoagulated as per the respective guidelines. Dronedarone can increase the bioavailability of dabigatran (1.7-fold increase in dabigatran absorption) and for this reason it is recommended that the drugs should not be combined (and particularly not with the 150 mg twice-daily dose of dabigatran). However, all patients, irrespective of treatment with a specific AAD, should be appropriately anticoagulated.
Serum creatinine levels
Changes in serum creatinine are used commonly to reflect renal function. However, dronedarone causes a small, predictable rise in creatinine9 (due to its effect on secretion rather than glomerular filtration). This rise is up to 10% and plateaus after 7–14 days. Taking baseline readings on initiation and testing again at one week and one month is sufficient to confirm that levels are stable (within 10% of the previous estimates). This schedule is convenient and coincides with liver function testing, but differs slightly from that recommended by the EMA. Amiodarone also leads to a rise of serum creatinine by an identical mechanism, but no regulatory recommendation has been made with regard to repeating or re-establishing a new baseline for creatinine concentration.
Conclusion
This position statement aims to provide professionals caring for patients with AF with clear and practical recommendations for the use and monitoring of AADs (in particular dronedarone) in the appropriate AF patients. All AADs are contraindicated in some patients, precautions are necessary in other conditions and monitoring requirements are needed to ensure that the right patients benefit.
The implications for clinical practice of recent regulatory changes have been debated and discussed by the expert group. Practical guidance on the clinical interpretation of these revisions is provided here to give HCPs confidence in the safe use of AADs, including dronedarone. In non-permanent AF patients, where maintenance of sinus rhythm is the management goal, dronedarone is an important option, but dronedarone should be discontinued when maintaining sinus rhythm is no longer appropriate (figure 1).

A variety of options now exist for treating and managing non-permanent AF, depending on the individual patient’s comorbidities and their response to a particular AAD. The more treatment choices available to clinicians, the more likely it is that they will be able to optimise therapy for the individual patient. Ensuring that HCPs have a full understanding of what constitutes the right treatment for the right patient, at the right time during the progression of the disease will benefit AF patients.
Conflict of interest
Sanofi supported the initial meeting of the expert group and the group’s travel expenses and honoraria. The consensus statement content and the opinions expressed, however, are those of the authors (the expert group) alone. Editorial assistance was supported by Sanofi and provided by Lucy Smithers of Interlace Global. The authors reviewed and approved the consensus statement before publication.
AJC is an advisor to Sanofi, Merck, BMS, Gilead, Menarini and Xention, and speaks on behalf of Sanofi, Merck, BMS, Gilead and Menarini. CA has received advisory board fees, speaker’s honoraria and research support from Sanofi. AMC has acted as a consultant for Pfizer, Sanofi and Merck, has received a research grant from St Jude Medical, an educational grant from Sanofi, and travel grants from Biotronik and Medtronic. RAK has acted as a consultant for Sanofi. DK is a consultant with Bard, Boston Scientific, Lakeshore Region Medical, Medtronic, and Sanofi, and has intellectual property agreements with Bard and Medtronic. KK has received advisory board fees and speaker’s honoraria from Sanofi. EL has received speaker’s honoraria from Sanofi and Boehringer-Ingelheim, and conference sponsorship from Medtronic. GYHL has served as a consultant for Bayer, Astellas, Merck, Sanofi, BMS/Pfizer, Daiichi-Sankyo, Biotronik, Portola and Boehringer-Ingelheim, and has been on the speakers’ bureau for Bayer, BMS/Pfizer, Boehringer-Ingelheim and Sanofi Aventis. FM has received advisory board fees from Sanofi, Medtronic Inc. and Boston Scientific, speaker’s honoraria from Boehringer-Ingelheim and Boston Scientific, and receives support for fellowships and research from Medtronic and Sorin. GAN has received advisory board fees and speaker’s honoraria from Sanofi. NP is an advisor to and speaks on behalf of Sanofi. HP has received lecturing and advisory board fees from Sanofi, Bayer, Boehringer-Ingelheim and Pfizer. PS and NS have acted as advisors to Sanofi. HW has received speaker’s honoraria and advisory board fees from Sanofi.
Key messages
- There is an urgent need for practical guidance on the use of anti-arrhythmic drugs (AADs), particularly in light of recent changes to regulatory recommendations for dronedarone, which this position statement aims to provide
- All AADs carry risks that must be weighed against the benefits they may provide, and all require regular monitoring for their safe use
- The evidence base supports the use of dronedarone for maintenance of sinus rhythm in non-permanent atrial fibrillation (AF) patients without severe heart failure, no previous lung or liver toxicity from amiodarone, and who are appropriately anticoagulated
- A range of treatment choices for non-permanent AF, including dronedarone, enables physicians to provide optimal therapy to each individual patient
References
- Camm AJ, Kirchhof P, Lip GY et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–429. http://dx.doi.org/10.1093/eurheartj/ehq278
- Skanes AC, Healey JS, Cairns JA et al. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol 2012;28:125–36. http://dx.doi.org/10.1016/j.cjca.2012.01.021
- Wann LS, Curtis AB, January CT et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011;57:223–42. http://dx.doi.org/10.1016/j.jacc.2010.10.001
- National Institute for Health and Clinical Excellence. TA197 Atrial fibrillation – dronedarone: guidance. London: NICE, August 2010. Available from: http://www.nice.org.uk/guidance/TA197/Guidance/pdf
- Freemantle N, Lafuente-Lafuente C, Mitchell S et al. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace 2011;13:329–45. http://dx.doi.org/10.1093/europace/euq450
- Hohnloser SH, Crijns HJ, van Eickels M et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009;360:668–78. http://dx.doi.org/10.1056/NEJMoa0803778
- Singh BN, Connolly SJ, Crijns HJ et al. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007;357:987–99. http://dx.doi.org/10.1056/NEJMoa054686
- Le Heuzey JY, De Ferrari GM, Radzik D et al. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol 2010;21:597–605. http://dx.doi.org/10.1111/j.1540-8167.2010.01764.x
- Davy JM, Herold M, Hoglund C et al. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the efficacy and safety of dronedarone for the control of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J 2008;156:527.e1–527.e9.
- Nieuwlaat R, Hohnloser SH, Connolly SJ. Effect of dronedarone in patients with permanent atrial fibrillation during the ATHENA study. Eur Heart J 2011;32:Abstract P3565.
- Connolly SJ, Camm AJ, Halperin JL et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011;365:2268–76. http://dx.doi.org/10.1056/NEJMoa1109867
- Khan K, Rivlin G. The appropriate use of amiodarone: lessons from practice. Cardiology News 2011;14:10–12.
- Waldo AL, Camm AJ, deRuyter H et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet 1996;348:7–12. http://dx.doi.org/10.1016/S0140-6736(96)02149-6
- Echt DS, Liebson PR, Mitchell LB et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 1991;324:781–8. http://dx.doi.org/10.1056/NEJM199103213241201
- Bardy GH, Lee KL, Mark DB et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. http://dx.doi.org/10.1056/NEJMoa043399
- Vorperian VR, Havighurst TC, Miller S, January CT. Adverse effects of low dose amiodarone: a meta-analysis. J Am Coll Cardiol 1997;30:791–8. http://dx.doi.org/10.1016/S0735-1097(97)00220-9
- Vassallo P, Trohman RG. Prescribing amiodarone: an evidence-based review of clinical indications. JAMA 2007;298:1312–22. http://dx.doi.org/10.1001/jama.298.11.1312
- Sanofi-Aventis. Multaq Summary of Product Characteristics, 2011;1–34.
