This study was designed to evaluate the impact of a novel iterative reconstruction (IR) algorithm on an established UK cardiac computerised tomography (CT) service. Areas assessed included image quality and effective radiation dose (ED).
A total of 250 consecutive patients with suspected coronary artery disease were enrolled as a substudy of a larger trial. Examinations were performed on a 64-channel detector CT with data sets reconstructed with the standard filtered back projection (FBP) or IR technique. Image noise was measured within predefined regions of interest (ROI), and image quality qualitatively assessed by two clinicians blinded to the reconstruction method. ED was calculated using a chest-specific conversion coefficient.
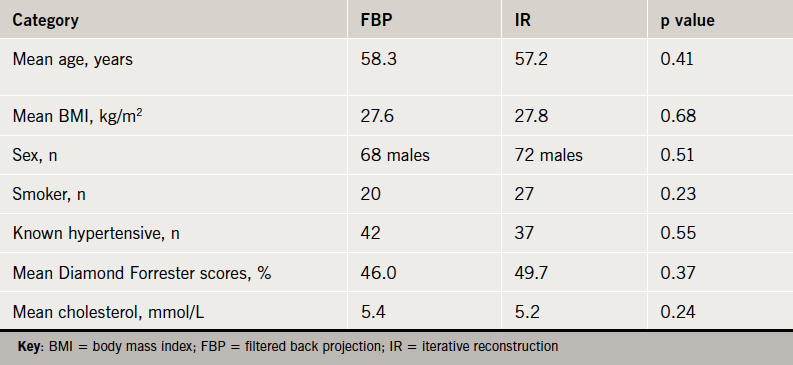
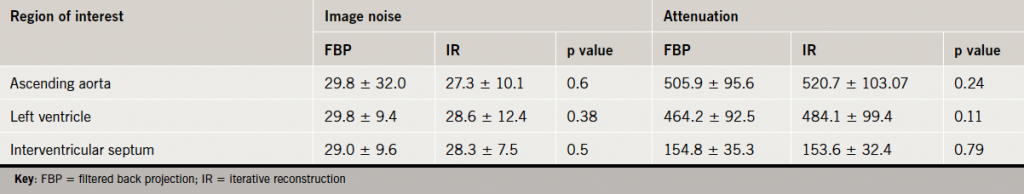
Four patients withdrew. So, 246 patients (140 males) underwent cardiac CT: 124 consecutive patients underwent a routine scanning protocol, with images reconstructed with FBP, and 122 patients with IR technique. The mean estimated EDs were 6.5 mSv (FBP) and 4.3 mSv (IR) (dose savings 34%) for all patients (p<0.00001). There was no statistical difference in noise or mean attenuation between the IR and FBP images. The mean IR image quality score was 3.67 ± 1.04 compared with 3.29 ± 1.17 for FBP images (p<0.001).
IR in cardiac CT offers substantial ED reduction without compromise in image quality.
Introduction
The use of cardiac computerised tomography (CT) in the UK is changing. National Institute for Health and Clinical Excellence (NICE) clinical guideline 95 (CG95) defined its role in the assessment of stable chest pain patients.1 Further, recent NICE diagnostics guidance 3 (DG3) has recommended the use of newer scanners for difficult patients and specifically addressed the concerns about the effective radiation dose (ED) of earlier CT platforms.2
However, the commercial availability of the latest CT scanners is not yet widespread within the National Health Service (NHS). The 64-detector CT is presently the workhorse of the NHS and is the recommended technological minimum for a new cardiac CT service. Recent meta-analyses have quoted its per-patient specificity for detecting >50% lesions as between 89 and 96% with sensitivity of 93 and 99%.3-6 In the absence of dedicated higher detector models, the 64 scanner will be the cornerstone for CG95 adoption in most institutions.
The past five years has witnessed unprecedented advances in ED reduction, with individualised protocol selection, retrospective tube dose modulation, bismuth breast shields7 and low-dose prospective axial electrocardiogram (ECG)-triggered gated image acquisition.8,9 More recently, the rebirth of iterative reconstruction (IR) techniques has been heralded as another significant development for cardiac CT image acquisition. IR is not a new concept. Initial reports by Brooks and Di Chiro appear in the literature as early as 1975.10 IR algorithms create more accurate final images by performing repeated ‘iterative’ reconstruction cycles on image data, reducing the amount of electronic noise. Although the concept was sound, helical CT systems lacked the ability to facilitate clinical IR adoption until recently.11,12
The purpose of this study was to assess the impact of the introduction of a novel hybrid IR platform on an established UK cardiac CT service. Outcome measures included image noise, diagnostic image quality and radiation exposure.
Methods
The CAPIR study (CT assessment of chest pain with iterative reconstruction) recruited patients that were enrolled in an ongoing current trial, the Cardiac CT for the Assessment of Pain and Plaque (CAPP) study [ISRCTN52480460]. The CAPP study is a randomised-controlled trial designed to evaluate the use of cardiac CT as a primary imaging test for patients attending a rapid access chest pain clinic (RACPC) within the UK. The study protocol was approved by the Office for Research Ethics Committee Northern Ireland (ORECNI) and the South Eastern Health and Social Care Trust (SEHSCT) Research and Development Committee.
Patients
The CAPIR study population consisted of the 250 patients with suspected CAD that had been randomised to the CT-imaging arm of CAPP. The exclusion criteria were: previous known coronary disease; a history of contrast media reaction; a body mass index greater than 35 kg/m2; tachyarrhythmias; impaired renal function with an estimated glomerular filtration rate (eGFR) of less than 35 ml/minute; severe aortic stenosis; acute myocarditis or pericarditis; uncontrolled hypertension >220/100 mmHg; severe peripheral vascular disease or impaired mobility; left bundle branch block; or any other clinical reason that the attending clinician thought would compromise the patient’s safety.
CT image acquisition
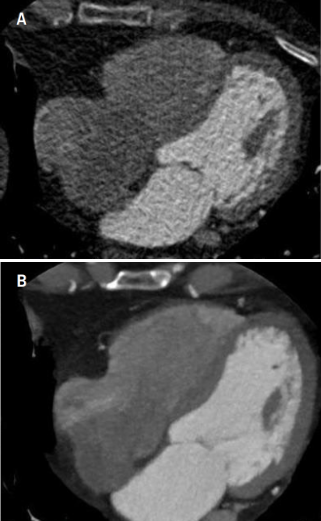
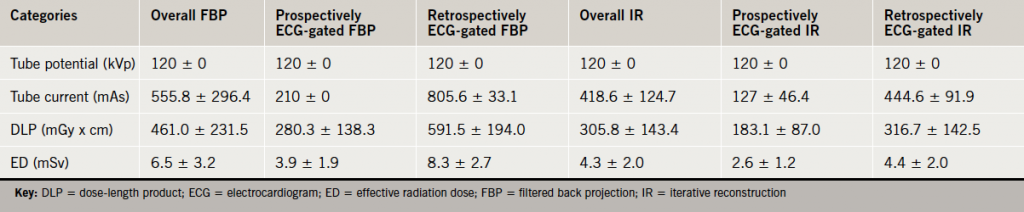
CT was performed on a first generation 64-channel scanner (Brilliance CT, Philips Healthcare, Cleveland, Ohio, USA). As per departmental policy, both oral and intravenous beta blockers were used for heart rate control prior to scanning, and targeted a rate below 65 beats per minute.
A non-contrast enhanced prospective axial calcium score (CS) was performed. Patients were allocated into two cohorts. Cohort A underwent CT coronary angiography (CTCA) using a standard protocol, with images reconstructed with a standard filtered back projection (FBP) technique. Cohort B underwent CTCA with images reconstructed with a novel IR technique, iDose4 (Philips Healthcare, Cleveland, Ohio, USA). The reduction in tube output for Cohort B was based on initial phantom study experience.13 All patients underwent a standardised 120 kV protocol. Other scan parameters (mAs, and scan-length) were optimised by the imaging clinician and were patient specific. The choice of retrospective or prospective ECG triggering (figure 1) was at the discretion of the clinician and influenced by factors such as the resting heart rate, heart rate variability, and pre-test likelihood of coronary artery disease (CAD). For all retrospective ECG-gated examinations, ECG dose modulation algorithms were applied (DoseRight Cardiac, Philips Healthcare, Cleveland, Ohio, USA).
Assessment of image quality
Each patient had their data anonymised and transferred to a remote workstation. Images were then assessed for noise and signal quality within circular regions of interest (ROIs) on axial images. Noise was defined as the standard deviation of the measured Hounsfield unit (HU), and signal as the HU mean attenuation value. The ROIs were in the ascending aorta, interventricular septum and left ventricular cavity.
Subjective image qualities were rated by an experienced cardiologist and radiologist in a blinded fashion using a five-point Likert scale. Images were scored according to the degree of image noise, quality of coronary contour delineation, general image impression, reconstruction artefact, and ease of diagnosis (1=non-diagnostic; 2=fair; 3=moderate; 4=good; 5=excellent).
The ED of each CTCA was estimated by multiplying the dose-length product (DLP) by a chest-specific conversion coefficient (κ=0.014 mSv×mGy−1×cm−1).14,15
Statistical analysis
Statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, Illinois, USA). Continuous variables are presented as mean ± standard deviation (SD) and compared using an independent t-test for normally distributed data. P values <0.05 were considered statistically significant for all data analyses. Inter-observer agreements for subjective image quality were quantified using kappa statistics.
Results
Patient demographics
A total of 250 patients were eligible for the CAPIR study. Four patients withdrew. The remaining 246 patients proceeded to have a CS followed by CTCA. Cohort A consisted of 124 patients who received a FBP protocol. Cohort B consisted of 122 patients who received an IR protocol. Of the 246 there were 140 males and 106 females. There were no significant differences between the two cohorts’ demographics (table 1).
Protocol selection
Of the 124 consecutive patients in the FBP cohort, 72 underwent a helical retrospectively ECG-gated protocol and 52 a prospectively ECG-triggered protocol. Of the 122 that received an IR study, 112 received a retrospective protocol and 10 a prospective.
Radiation dose estimates
The mean ED of Cohort A was 6.5 mSv. Cohort B had a lower mean ED of 4.3 mSv, thus, representing dose savings of 2.2 mSv (33.6%) (p<0.00001) (table 2). The mean ED for FBP retrospectively ECG-gated studies in Cohort A was 8.3 mSv, with an equivalent mean ED in Cohort B of 4.4 mSv. IR appeared to provide a mean dose saving of 3.9 mSv or 46.5% dose reduction for retrospectively ECG-gated examinations.
within cohorts, mean ± standard deviation
Image noise, attenuation and image quality
There was no statistical difference in noise or mean attenuation between the IR and FBP images in all three ROI (table 3). The observers’ image quality scores were similar for both IR and FBP scans, with Kappa coefficients of 0.82 and 0.84, respectively. The mean image quality score obtained from the IR images was 3.7 ± 1.0 compared with the FBP images of 3.3 ± 1.2, which was statistically different with a p value of 0.0067 (figure 1).
Discussion
DG3 emphasised the importance of low radiation dose diagnostic cardiac CT examinations. Adoption of DG3 in the NHS will be determined by the availability of the latest generation of cardiac platforms. This study has identified some benefits of IR introduction to an established NHS cardiac CT service using a first generation 64-detector platform, without the need for substantial investment.
The last 10 years has witnessed an unprecedented growth in diagnostic CT imaging. Cardiovascular imaging represents at least a third of the medical imaging examinations performed annually worldwide.16 Between 1993 and 2002, cardiovascular imaging grew more than twice as rapidly as medical imaging for non-cardiovascular disease.17 At present, CT is the single greatest source of medical radiation exposure.18 Iatrogenic CT exposure is thought to contribute to 2.0% of all cancers.18 Recent multi-centre, multi-vendor studies have highlighted the importance of radiation dose reduction, the potential carcinogenic effects, and the importance of adherence to the ALARA (as low as reasonably achievable) principle in cardiac CT imaging.19,20
Guidelines from the American Heart Association21 suggested that an ED of 10 mSv increases the lifetime risk of a fatal malignancy by approximately 0.05%. Therefore, it is the responsibility of all clinicians to reduce this risk to an acceptable level without image quality degradation. In addition, clinicians must be mindful of the cumulative radiation exposure a patient may receive in the investigation of chest pain. Myocardial perfusion imaging with a two-day stress–rest Technetium protocol typically results in an ED of 10–15 mSv,22,23 and invasive coronary angiogram 4–7 mSv.24,25
There have been a number of studies published on the use of IR in cardiac CT imaging. These have highlighted the theoretical benefits of retrospective IR reconstruction to FBP data within the image domain.26-28 To our knowledge, this is the first study to prospectively compare FBP and IR in consecutive patients undergoing cardiac CT for the assessment of stable chest pain.
This study has a number of limitations. First, it was not a randomised-controlled trial, but two distinct cohorts of consecutive patients that underwent CTCA for the assessment of chest pain. Second, there were a number of exclusion criteria, which excluded a number of patients from the study. Third, image quality assessment was based on a subjective Likert score. Fourth, there were fewer prospective ECG-triggered exams in the IR group. Despite this, cohort B had a significantly lower radiation dose. It is likely that the adaption of IR to axial prospective gated protocols with associated ‘ECG padding’ could convey substantial further dose reduction and maintain diagnostic accuracy. Finally, 100 kV imaging was not commercially available at the time of study design and initiation. Consequently, all patients underwent a standardised 120 kV protocol. It is likely that the routine application of 100 kV imaging in appropriate patients would have yielded further substantial dose savings.
The key to successful cardiac CT is image quality. This study has demonstrated that the application of IR algorithms for cardiac CT can offer substantial radiation dose reductions without image quality compromise, on existing cardiac CT platforms.
Funding
This work was supported by the South Eastern Health and Social Care Trust [SET/10/52].
Conflict of interest
None declared.
Key messages
- The key to successful cardiac imaging must balance diagnostic image quality with the lowest possible radiation exposure
- The as low as reasonably achievable (ALARA) principle must be adhered to at all times. However, accurate initial diagnostic imaging quality is vital to prevent cumulative radiation doses from multiple tests
- In cardiac CT, all operators have a responsibility to reduce radiation doses through individualised optimisation of scan protocols (kV, mAs, tube dose modulation)
- Iterative reconstruction is a novel technique that allows a significant further reduction in radiation dose without compromise in image quality or noise
References
- Skinner JS, Smeeth L, Kendall JM et al. NICE guidance. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. Heart 2010;96:974–8. http://dx.doi.org/10.1136/hrt.2009.190066
- National Institute for Health and Clinical Excellence (NICE). New generation cardiac CT scanners (Aquilion ONE, Brilliance iCT, Discovery CT750 HD and Somatom Definition Flash) for cardiac imaging in people with suspected or known coronary artery disease in whom imaging is difficult with earlier generation CT scanners. London: NICE, December 2012. Available from: http://guidance.nice.org.uk/DG3/Guidance/pdf/English [accessed 20 December 2012].
- Salavati A, Radmanesh F, Heidari K et al. Dual-source computed tomography angiography for diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. J Cardiovasc Comput Tomogr 2012;6:78–90. http://dx.doi.org/10.1016/j.jcct.2011.10.018
- Takakuwa KM, Keith SW, Estepa AT, Shofer FS. A meta-analysis of 64-section coronary CT angiography findings for predicting 30-day major adverse cardiac events in patients presenting with symptoms suggestive of acute coronary syndrome. Acad Radiol 2011;18:1522–8. http://dx.doi.org/10.1016/j.acra.2011.08.013
- Mowatt G, Cook JA, Hillis GS et al. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart 2008;94:1386–93. http://dx.doi.org/10.1136/hrt.2008.145292
- Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R et al. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology 2007;244:419–28. http://dx.doi.org/10.1148/radiol.2442061218
- Yilmaz MH, Yasar D, Albayram S et al. Coronary calcium scoring with MDCT: the radiation dose to the breast and the effectiveness of bismuth breast shield. Eur J Radiol 2007;61:139–43. http://dx.doi.org/10.1016/j.ejrad.2006.08.012
- Kalra MK, Maher MM, Toth TL et al. Strategies for CT radiation dose optimization. Radiology 2004;230:619–28. http://dx.doi.org/10.1148/radiol.2303021726
- Paul JF, Abada H. Strategies for reduction of radiation dose in cardiac multislice CT. Eur Radiol 2007;17:2028–37. http://dx.doi.org/10.1007/s00330-007-0584-3
- Brooks RA, Di Chiro G. Theory of image reconstruction in computed tomography. Radiology 1975;117:561–72.
- Sagara Y, Hara AK, Pavlicek W. Abdominal CT: comparison of low-dose CT with adaptive statistical iterative reconstruction and routine-dose CT with filtered back projection in 53 patients. Am J Roentgenol 2010;195:713–19. http://dx.doi.org/10.2214/AJR.09.2989
- Prakash P, Kalra MK, Ackman JB et al. Diffuse lung disease: CT of the chest with adaptive statistical iterative reconstruction technique. Radiology 2010;256:261–9. http://dx.doi.org/10.1148/radiol.10091487
- Noël PB, Fingerle AA, Renger B et al. Initial performance characterization of a clinical noise-suppressing reconstruction algorithm for MDCT. Am J Roentgenol 2011;197:1404–09. http://dx.doi.org/10.2214/AJR.11.6907
- Bischoff B, Hein F, Meyer T et al. Comparison of sequential and helical scanning for radiation dose and image quality: results of the Prospective Multicenter Study on Radiation Dose Estimates of Cardiac CT Angiography (PROTECTION) I study. Am J Roentgenol 2010;194:1495–9. http://dx.doi.org/10.2214/AJR.09.3543
- Bongartz G, Golding SJ, Jurik AG et al. European guidelines for multislice computed tomography. Brussels, Belgium: European Commission, 2004; FIGM-CT2000-20078-CT-TIP.
- Picano E. Economic and biological costs of cardiac imaging. Cardiovasc Ultrasound 2005;3:13. http://dx.doi.org/10.1186/1476-7120-3-13
- Levin DC, Rao VM, Parker L et al. Recent trends in utilization of cardiovascular imaging: how important are they for radiology? J Am Coll Radiol 2005;2:736–9. http://dx.doi.org/10.1016/j.jacr.2005.01.015
- Brenner DJ, Hall EJ. Computed tomography – an increasing source of radiation exposure. N Engl J Med 2007;357:2277–84. http://dx.doi.org/10.1056/NEJMra072149
- Einstein AJ, Moser KW, Thompson RC et al. Radiation dose to patients from cardiac diagnostic imaging. Circulation 2007;116:1290–305. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.688101
- Hausleiter J, Meyer T, Hermann F et al. Estimated radiation dose associated with cardiac CT angiography. JAMA 2009;301:500–07. http://dx.doi.org/10.1001/jama.2009.54
- Gerber TC, Carr JJ Arai AE et al. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation 2009;119:1056–65. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.191650
- Einstein AJ. Radiation risk from coronary artery disease imaging: how do different diagnostic tests compare? Heart 2008;94:1519–21. http://dx.doi.org/10.1136/hrt.2007.135731
- Berrington de Gonzalez A, Kim K-P, Smith-Bindman R, McAreavey D. Myocardial perfusion scans. Projected population cancer risks from current levels of use in the United States. Circulation 2010;122:2403–10. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.941625
- Anonymous. Sources and effects of ionizing radiation. United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2000 report to the General Assembly, with scientific annexes. New York: United Nations, 2000. Available from: http://www.unscear.org/unscear/en/publications/2000_1.html [accessed 20 December 2012].
- Vijayalakshmi K, Kelly D, Chapple CL et al. Cardiac catheterisation: radiation doses and lifetime risk of malignancy. Heart 2007;93:370–1. http://dx.doi.org/10.1136/hrt.2006.098731
- Gosling O, Loader R, Venables P et al. A comparison of radiation doses between state-of-the-art multislice CT coronary angiography with iterative reconstruction, multislice CT coronary angiography with standard filtered back-projection and invasive diagnostic coronary angiography. Heart 2010;96:922–6. http://dx.doi.org/10.1136/hrt.2010.195909
- Renker M, Nance JW Jr, Schoepf UJ et al. Evaluation of heavily calcified vessels with coronary CT angiography: comparison of iterative and filtered back projection image reconstruction. Radiology 2011;260:390–9. http://dx.doi.org/10.1148/radiol.11103574
- Moscariello A, Takx RA, Schoepf UJ et al. Coronary CT angiography: image quality, diagnostic accuracy, and potential for radiation dose reduction using a novel iterative image reconstruction technique – comparison with traditional filtered back projection. Eur Radiol 2011;21:2130–8. http://dx.doi.org/10.1007/s00330-011-2164-9
