This year’s 8th Annual Scientific Meeting of the Cardiorenal Forum looked at clinical dilemmas in cardiorenal disease and co-morbidities. The meeting was held at the Royal College of Obstetricians and Gynaecologists, London, October 4th 2013, and endorsed by the Renal Association and the British Society for Heart Failure. Alice Zheng and Katy Watson report on its highlights.
Advances in imaging and diagnosis
Dr Nik Abidin (Consultant Cardiologist, Salford Royal NHS Foundation Trust) kicked off the theme of ‘Advances in diagnosis’ with a tantalising taster of the future of echocardiography, and a demonstration of what is already possible. Patients with chronic kidney disease (CKD) have a high incidence of cardiac dysfunction, with 75% of patients with significant CKD demonstrating left ventricular hypertrophy. In such patients, left ventricular dilatation occurs late with advanced disease, and left ventricular mass is an earlier predictor of cardiac mortality. An increase in left atrium size is the downstream effect of left ventricular dysfunction and is also associated with worse outcome.
Two-dimensional (2D) echocardiography relies on linear beam oscillation to measure ventricular dimensions from still 2D images to calculate ejection fraction (EF). Conventional methods of measurement and calculation e.g. Simpson’s method, or using M-mode measurements have inherent limitations and result in different EFs, allowing only a range of either mild, moderate or severe dysfunction to be reported. This lack of accuracy and reproducibility makes the follow-up of a particular patient throughout treatment difficult. Left ventricular mass can be calculated using measurements from both M-mode and 2D images, incorporated into various mathematical formulae, all based on a spherical geometrical assumption of the left ventricle, and all resulting in different values.
Three-dimensional (3D) echocardiography uses a matrix array ultrasound probe, allowing acquisition of multiple ‘pyramid’ volumes of data (see figures 1a–1d). Once processed, the virtual 3D heart can be sliced and analysed from infinite planes, allowing for the non-geometrical shape of the ventricles. Volume, particularly at the apex, traditionally lost by 2D echocardiography techniques can also be accounted for. It has been demonstrated that using 3D echocardiography to trace the epicardial and endocardial borders in different planes, an individual’s calculated left ventricular mass can be halfof that calculated using 2D techniques.
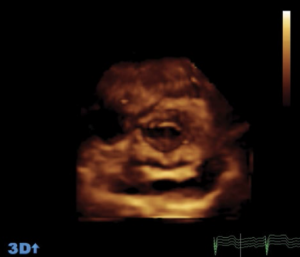
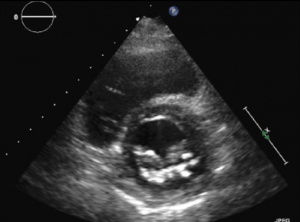
Cardiac magnetic resonance imaging (MRI) is currently the gold standard for assessing cardiac anatomy, function and mass. Jenkins et al.1looked into the reproducibility and accuracy of echocardiography and found little difference between 3D echocardiography and cardiac MRI-measured ventricular volumes, whereas 2D echocardiography gave a variability of up to 50 ml, which potentially could alter the severity class of dysfunction. 3D echocardiography demonstrated consistently narrower variability and more reproducible results when analysed for both inter- and intra-operator variance. Limitations common to 2D echocardiography include the impairment of acoustic windows such as in patients with raised body mass index (BMI) or lung disease, i.e. chronic obstructive pulmonary disease (COPD). 3D techniques are extremely useful in the assessment of valves and foreign lines e.g. pacing wires in relation to the surrounding structures. Most machines already have 3D echocardiography capabilities, and once familiarised with the technique 3D scans are quicker to carry out than the conventional 2D technique.
3D echocardiography is new and exciting; due to its accuracy and reproducibility it has significant applicability in the research field, adding power to studies. It is a valuable assessment tool which we will be seeing more of in the future.
Advances in treatments
Renal denervation is a novel treatment for resistant hypertension. It is a minimally invasive, endovascular catheter-based procedure which uses radiofrequency ablation to target the sympathetic nerves in the adventitia of the renal arteries. The ablation is performed in a swirl pattern with a series of burns (see figure 2), thereby preventing a circular burn which theoretically could result in renal artery stenosis. Resistant hypertension is notoriously difficult to treat, often affects the younger person, and carries a high morbidity from stroke, heart failure (HF) and progressive renal impairment. Renal denervation is currently being performed at certain centres around the UK. The outcomes of the SYMPLICITY trials, and his own clinical experience, were presented by Dr Hitesh Patel, an SpR at the Royal Brompton Hospital, London.
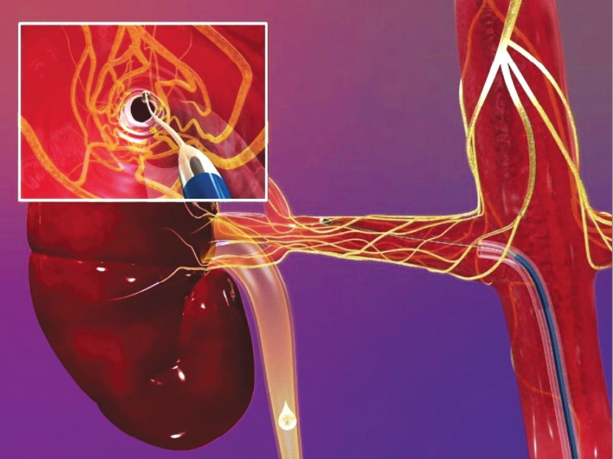
SYMPLICITY I and II have shown very promising results at 36 months, with a mean systolic blood pressure (BP) reduction of 33 mmHg.2 Equal benefit has been seen amongst those with normal and mildly impaired renal function; however trials to date have excluded patients with an estimated glomerular filtration rate (eGFR) <45 ml/min/1.73 cm2. SYMPLICITY I and II demonstrated from subgroup analysis that this technique was only beneficial in individuals with ambulatory BP satisfying the criteria of resistant BP (i.e. BP ≥160 mmHg systolic despite at least three antihypertensive agents, including a diuretic at maximal tolerated doses).3 These early trials, however, have not been powered for hard cardiovascular end points, and had only 36 months of patient follow-up.
The experience at the Brompton has been of patients tolerating the procedure well, with a mean admission of 24 hours. Complication rates have been low with no renal artery thrombosis. Future studies will allow for a longer follow-up and will look to include individuals with more advanced CKD.
Dr Patel introduced future studies at the Brompton & Harefield Trust with the National Institute for Health Research (NIHR) Cardiovascular Biomedical Research, which will investigate the role of renal denervation in individuals with HF and preserved EF. This is a hard-to-manage patient group with a minimal existing evidence base. Recruitment for this trial started July 2013 and preliminary findings have shown a significant reduction in left ventricular mass, left atrial (LA) volume and left ventricular filling pressure (E/E) at six months post-renal denervation.4 This study excludes individuals with an eGFR <45 ml/min/1.73 m2, contraindications to cardiac MRI, cardiomyopathy or unfavourable renal artery anatomy. Early results are promising, and we can hold out hope for this novel therapy.
Cardiorenal syndrome (CRS) is a major clinical problem for cardiologists and nephrologists, and is an indicator of poor prognosis in individuals with HF which itself carries a high mortality. Currently there is no clear evidence base with which to guide treatment. Novel approaches include renal denervation. Professor Henry Krum (Monash University, Melbourne, Australia) illustrated that in animal models with CRS, renal blood flow and renal vascular resistance improves post-renal denervation.5 SYMPLICITY-HF is a novel trial looking to investigate the role of renal denervation in systolic HF and co-morbid CKD stage II and III. This landmark trial will include those with New York Heart Association (NYHA) Class II–III, a left ventricular EF <40%, eGFR 30–75 ml ml/min/1.73 m2 and on optimal medical therapy.
Trials performed in the cardiorenal population and shortfalls
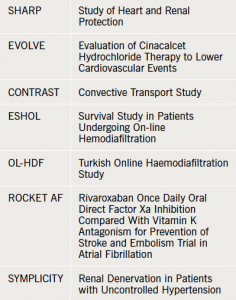
Cardiorenal medicine lacks well-powered trials. Renal dysfunction is a common exclusion criterion from landmark cardiovascular studies, leaving us with dilemmas in managing our patients. Professor David Goldsmith, (Consultant Nephrologist, Guy’s and St Thomas’ NHS Foundation Hospital, London) presented data from recent trials and highlighted the shortfalls and surprise findings. These included the controversial SHARP study, EVOLVE, and the dialysis trials CONTRAST, OL-HDF and ESHOL (see table 1).
SHARP looked at cardiovascular outcomes in patients treated with simvastatin/ezetimibe versus placebo in the CKD and dialysis population.6 While benefit was seen in CKD, similar to that in the general population, those on dialysis failed to show any benefit. This potentially highlights the different pathogenesis in cardiovascular disease in those on dialysis where the haemodynamic stress of dialysis itself outweighs the traditional risk factors, but it may be a shortfall of the study with this subgroup being underpowered.
EVOLVE looked at cinacalcet in the treatment of secondary hyperparathyroidism in dialysis patients.7 Secondary hyperparathyroidism itself, and its more traditional treatments, result in abnormalities in calcium and phosphate metabolism. High calcium phosphate product is associated with an increased risk for all-cause mortality and cardiovascular morbidity in the dialysis population. This mechanism is likely to be mediated by increased vascular calcification, increased arterial stiffness and left ventricular hypertrophy. This was a landmark trial being the first well-powered randomised control trial focussing on the dialysis population. The trial showed that cinacalcet, a calcimimetic, reduced parathyroid hormone (PTH) successfully but this did not translate to any improvement in cardiovascular outcomes, or any reduction in all-cause mortality. Disappointingly there were inadequacies in the trial design. The placebo group was permitted to continue with “flexible use of traditional therapies” to manage the hyperparathyroidism. Unfortunately, with the more widespread availability of cinacalcet, both arms of the trial were being given the drug, i.e. masking any benefit of the treatment arm and invalidating the outcome.
The three large trials comparing online haemodiafiltration to standard haemodialysis were discussed, the theory being that the more expensive haemodiafiltration enables more efficient removal of beta 2 microglobulin and other non-uraemic toxins than standard dialysis therapy, and would result in less cardiovascular morbidity. However, CONTRAST and OL-HDF failed to show any difference between primary end points despite the surrogate end point showing significant reduction of beta 2 microglobulin. ESHOL did see some benefit but this was small and deemed insufficient to outweigh the increased expense.
This session highlighted the need in cardiorenal medicine for more well-designed and well-powered randomised control trials.
Future promising areas for research
Dr Paul Cockwell (Consultant Nephrologist, Renal Institute of Birmingham) introduced the theme of advancing research in cardiorenal medicine. His work has focussed on serum-free light chains and its use as a prognostic indicator in the progression of CKD. Malignant proliferation with raised monoclonal serum light chains is seen in the plasma cell dyscrasia- light chain myeloma. When a threshold concentration of light chains is reached, overwhelming the renal tubular capacity to reabsorb these proteins, there is overspill into the urine. This phenomenon was first described by Dr Henry Bence Jones in 1847 and detection of light chains in the urine is a technique still used in the diagnosis of myeloma today. The monoclonal element can occur in both the kappa and lambda moiety leading to light chain deposition and is a common treatable cause of renal disease and end-stage renal failure.
In the absence of myeloma, in the context of inflammation or infection, titres of light chains rise. This rise is polyclonal and the ratio of kappa to lambda is unchanged. Light chains, are, in part, renally excreted with 40% kappa and 20% lambda renal clearance respectively; the remainder are cleared by the reticulo-endothelial system. In CKD, renal clearance of light chains is reduced. Increased titres of polyclonalfree light chains in the general population without plasma cell disorders have been shown by both The Mayo Clinic and the UK Haematological Referral Population to be associated with a clear increase in all-cause mortality. Dr Cockwell presented study data from three cohorts of CKD patients from Birmingham, Salford and Derby. Increased serum polyclonal free light chains were shown to be independently associated with all-cause mortality in all three cohorts, and also associated with progression of CKD to renal replacement therapy (RRT).
Serum-free light chain analysis proves to be an exciting area of immunological diagnosis with a possible future role as a biomarker to predict the risk of progression of renal impairment in CKD.
Dilemmas: treating nonvalvular atrial fibrillation in CKD
The important frequently encountered scenario of appropriate anticoagulation in individuals with atrial fibrillation (AF) and CKD was debated at the forum. AF is extremely common with a prevalence of 2% in the general population but is 10–20 times more prevalent in CKD patients. The European Society of Cardiology (ESC) issued new AF guidelines in 20128 which shifted the focus from identifying high-risk patients to identifying truly low-risk patients, i.e. those not requiring anticoagulation. It recommended the use of CHA2DS2Vasc score for thromboembolic risk assessment and the HASBLED score for bleeding risk assessment in AF. Renal impairment was, however, an exclusion criterion in clinical trials and hence risk prediction scores are not validated in CKD. Patients with CKD and AF have a higher risk of thrombotic and bleeding complications. The risk of both complications is greater in dialysis-dependent individuals.
There have been attempts to assess whether renal function itself adds to risk prediction of stroke in AF. The ROCKET AF (rivaroxaban vs. warfarin) cohort was used to validate a new risk score R2CHADS2, involving the addition of 2 points for CrCl <60 ml/min, and identified a hazard ratio (HR) of 1.085.9 Unfortunately the trial cohort was not representative and did not include a range of renal dysfunction. Conclusions from this trial were that renal impairment does not add predictive value to existing risk scores. Deteriorating renal function, however, is a strong marker of poor outcome. Guo et al. found a relative reduction in eGFR of ≥25% over two years to independently predict the risk for ischaemic stroke or death at six months.10
Warfarin has been shown to decrease stroke risk significantly in CKD patients including those on dialysis, but aspirin alone does not. Anticoagulation and aspirin both increase the risk of bleeding, particularly in patients requiring dialysis. Trials with novel anticoagulants excluded those with advanced CKD eGFR <30 ml/min/1.73m2 and aren’t licensed in this population.
Dr Amitava Banerjee (Honorary Specialist Registrar, City Hospital, Birmingham) reiterated the need for further studies of change in renal function and clinical outcomes, as well as the need for trials of novel anticoagulants in renal failure. The management of AF and the decision to anticoagulate in CKD patients, especially those on RRT, remains complex given the paucity of evidence and known significant complications, including increased bleeding risk, and calciphylaxis with use of the coumarin agents. Pending further trials, the consensus of opinion from the meeting was to carefully consider each case on an individual basis.
Future
There are still many unknowns and uncertainties in the treatment of the high-risk cardiorenal population. This year’s forum has highlighted the shortfalls, and shed light on promising future areas of progress
Dr Kathryn Watson
Specialist Registrar, Renal Medicine
(kathyrn.watson@porthosp.nhs.uk)
Dr Alice Zheng
Senior House Officer, Cardiology
Queen Alexandra Hospital, Portsmouth, PO6 3LY
References
1. Jenkins C, Chan J, Bricknell K, Strudwick M, Marwick TH. Reproducibility of right ventricular volumes and ejection fraction using real-time three-dimensional echocardiography: comparison with cardiac MRI. Chest 2007;131:1844– 51. http://dx.doi.org/10.1378/ chest.06-2143
2. Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for treatment of drugresistant hypertension: one-year results from the Symplicity HTN- 2 randomized, controlled trial. Circulation 2012;126:2976–82. http://dx.doi.org/10.1161/ CIRCULATIONAHA.112.130880
3. Mahfoud F, Ukena C, Schmieder RE, et al. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation 2013;128:132–40. http://dx.doi.org/10.1161/ CIRCULATIONAHA.112.000949
4. Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012;59:901–9. http://dx.doi.org/10.1016/j. jacc.2011.11.034
5. Clayton SC, Haack KK, Zucker IH. Renal denervation modulates angiotensin receptor expression in the renal cortex of rabbits with chronic heart failure. Am J Physiol-Renal 2011;300:F31–9. http://dx.doi. org/10.1152/ajprenal.00088.2010
6. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92. http://dx.doi. org/10.1016/S0140-6736(11)60739- 3
7. Investigators ET, Chertow GM, Block GA, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. New Eng J Med 2012;367:2482–94. http://dx.doi. org/10.1056/NEJMoa1205624
8. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Euro Heart J 2012;33:2719–47. http://dx.doi. org/10.1093/eurheartj/ehs253
9. Piccini JP, Stevens SR, Chang Y, et al. Response to letter regarding article, “renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R2CHADS2 index in the ROCKET AF (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study cohorts”. Circulation 2013;128:e172–3. http://dx.doi.org/10.1161/ CIRCULATIONAHA.113.004013
10. Guo Y, Wang H, Zhao X, et al. Sequential changes in renal function and the risk of stroke and death in patients with atrial fibrillation. Int J Cardiol 2013;168:4678-84. http://dx.doi. org/10.1016/j.ijcard.2013.07.179


