This single-centre, retrospective, cohort study aims to provide insight into the long-term survival of patients ≥85 years old undergoing percutaneous coronary intervention (PCI) over a four-year observational period in a high-volume PCI centre. Between 2006 and 2010, 294 patients (mean age 88 ± 2 years, 56% male) underwent PCI at our institute. A total of 180 patients (61.2%) had an acute coronary syndrome (ACS) defined as unstable angina, non-ST elevation myocardial infarction (NSTEMI) or ST-elevation myocardial infarction (STEMI). One hundred and fourteen patients underwent PCI electively (38.8%).
The primary outcome was all-cause 30-day and one-year mortality rates. In-hospital, 30-day and one-year mortality rates were 2.4% (7 patients), 4.4% (13 patients) and 17.7% (52 patients), respectively, in the entire cohort. In addition, 30-day (5.6% vs. 3.4%, p=0.24) and one-year (20.0% vs. 14.0%, p=0.19) mortality rates were similar between the ACS and elective patients, respectively. Following multi-variable analysis, age (hazard ratio [HR] 1.14, 95% confidence interval [CI] 1.04 to 1.26), male sex (HR 1.85, 95% CI 1.01 to 3.42), previous PCI (HR 2.74, 95% CI 1.36 to 5.56) and the presence of shock (HR 15.39, 95% CI 6.67 to 35.50) emerged as independent predictors of one-year mortality rates.
We conclude that PCI appears to be a safe treatment option in very elderly patients with good one-year survival rates. Future randomised-controlled trials should specifically include this age group to guide interventional cardiologists in making decisions when faced with this very challenging cohort.
Introduction
 Over the last several years, the UK has witnessed a gradual ageing of its population.1 Moreover, the proportion of the very elderly (≥85 years old) in the general population is expected to rise fastest with a three-fold increase by the year 2035.1 Advancing age is perhaps the strongest predictor of de novo cardiovascular disease (CVD).2 As a consequence, cardiovascular (CV) mortality rates demonstrate a linear association with increasing age beyond the seventh decade. For example, octogenarians have a 10-fold greater risk of developing CVD in comparison with patients <50 years of age.2 Furthermore, mortality rates from CVD are higher for the very elderly, irrespective of clinical presentation.
Over the last several years, the UK has witnessed a gradual ageing of its population.1 Moreover, the proportion of the very elderly (≥85 years old) in the general population is expected to rise fastest with a three-fold increase by the year 2035.1 Advancing age is perhaps the strongest predictor of de novo cardiovascular disease (CVD).2 As a consequence, cardiovascular (CV) mortality rates demonstrate a linear association with increasing age beyond the seventh decade. For example, octogenarians have a 10-fold greater risk of developing CVD in comparison with patients <50 years of age.2 Furthermore, mortality rates from CVD are higher for the very elderly, irrespective of clinical presentation.
Following an acute coronary syndrome (ACS) with either ST elevation or non-ST elevation myocardial infarction (STEMI or NSTEMI), 30-day mortality rates in the very elderly have been shown to be 10-fold higher compared with patients <65 years old.3,4 In the setting of chronic stable angina, the Trial of Invasive versus Medica therapy in Elderly patients with chronic angina (TIME) demonstrated that patients managed medically had a two-fold increased risk of an adverse CV outcome compared with those undergoing myocardial revascularisation.5
Although the very elderly are at an increased CV mortality risk, evidence suggests that the proportion of patients who receive standard secondary prevention or revascularisation declines as they age beyond the eighth decade, despite both therapies being of proven prognostic benefit in this age group.6 Consequently, the American Heart Association (AHA) has developed guidelines to highlight the importance of these treatment options in the very elderly.4 As a result, new emerging evidence suggests that the proportion of very elderly patients undergoing percutaneous coronary intervention (PCI) is increasing.3,7 For example, two very large US registries have observed that up to one in nine patients undergoing PCI can be expected to be ≥85 years old.6,8 Paradoxically, there is clearly a paucity of very elderly patients included in major landmark PCI trials, which have historically underrepresented this age group (comprising ≤2% of the study population).4 Furthermore, the very elderly patients that have been included in randomised-controlled trials have traditionally had fewer comorbidities in comparison with their counterparts from registries and retrospective studies.4
In the absence of robust randomised clinical data on PCI treatment strategies for the very elderly cohort, observational studies remain valuable in providing insights to outcome and mortality trends. The purpose of this four-year retrospective analysis of patients ≥85 years treated with PCI at our centre was to demonstrate safety and efficacy of therapies and examine for predictors of mortality.
Methods
Study cohort
Between 1 July 2006 and 30 June 2010, a total of 6,398 patients were treated with PCI at our institute, currently the highest volume non-surgical PCI centre in the UK. Two hundred and ninety-four patients were aged 85 years or over (4.6%). This group was subdivided by mode of clinical presentation.
The ACS group comprised those patients with STEMI, NSTEMI or unstable angina. Patients were diagnosed with STEMI in the presence of new ST-segment elevation ≥1 mm seen in any location or new left bundle branch block (on index or subsequent electrocardiograms [ECGs]) with at least one elevated biochemical marker of myocardial necrosis (including troponin measurements). Patients with NSTEMI were diagnosed with ECG evidence of myocardial ischaemia (without ST-segment elevation) and biochemical evidence of cardiac necrosis. Finally, unstable angina was diagnosed with a history consistent with ACS and normal biochemical markers of myocardial necrosis. The elective group comprised patients with chronic stable angina who had symptoms despite two anti-anginal agents and, in addition, had demonstrable ischaemia with either fractional flow reserve (FFR) <0.80 or a positive non-invasive test (adenosine-stress magnetic resonance imaging [MRI], stress echocardiography or myocardial perfusion scintigraphy).
Cardiogenic shock patients were included in the ACS group and were defined according to the criteria used in the SHould we emergently revascularise Occluded Coronaries for cardiogenic shocK (SHOCK) trial.9 Standardised international definitions of all patient-related clinical diagnoses, complications and outcomes were adhered to. All patients undergoing PCI received heparin, aspirin and clopidogrel in accordance with current guidelines.10,11 Procedural decisions including access site, device selection, use of adjunctive pharmacotherapy and type of stent were left at the operator’s discretion. For patients undergoing ‘staged’ procedures, whereby they returned for an elective PCI having had the culprit vessel previously treated, only the index episode was included. All baseline clinical, angiographic and procedural data were entered into a dedicated electronic database by trained cardiac catheterisation laboratory physiologists and interventional cardiologists. All patients were established on standard secondary prevention therapy as recommended by guidelines at discharge.10,11 All patients were prospectively followed up for a minimum of one year through a specialised cardiac rehabilitation service and post-PCI clinics.
Primary outcome
The primary outcome measure for the present study was all-cause 30-day and one-year mortality rates.
Statistical analyses
All statistical analyses were performed using SPSS statistical software, version 19.0 (IBM, Armonk, NY, USA). Categorial variables are presented as number and percentage while continuous variables are presented as mean ± standard deviation (SD). An independent t-test was used to compare baseline characteristics between the ACS and elective groups with a significance level of p<0.05. Cumulative event rates of all-cause mortality were analysed using the Kaplan-Meier method. We used Cox proportional hazards regression to identify independent predictors of mortality for the entire cohort. Variables were presented with hazard ratios (HR) and 95% confidence intervals (CI). Following uni-variable analyses, we identified potential predictors of mortality (variables with a p value <0.1), which were subsequently jointly included in a multi-variable analysis to identify independent predictors of mortality presented as HR and 95% CI. A p value <0.05 was considered significant.
Results
Baseline patient characteristics
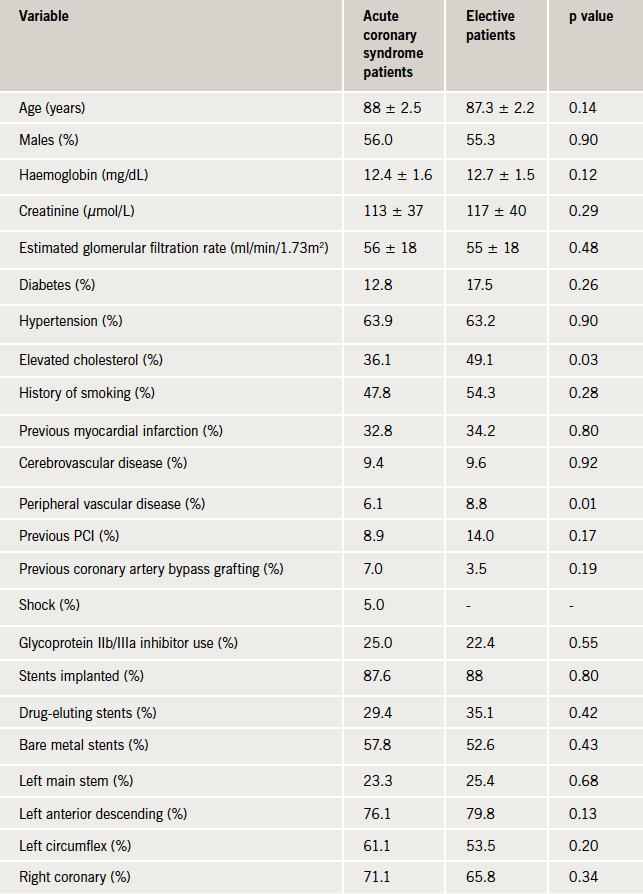
Baseline clinical and angiographic data are presented in table 1. The ACS group comprised 180 patients (61.2%) of which nine patients presented with cardiogenic shock. The elective group contained 114 patients (38.8%). There were no statistically significant differences in the baseline characteristics of the two groups other than a slightly higher proportion of the elective group having a history of elevated cholesterol and peripheral vascular disease.
Of the whole cohort, mean age was 88 ± 2 years, with the oldest patient treated aged 98 years. Within the ACS group, the percentage of patients that underwent an angiogram following STEMI and NSTEMI (or unstable angina) was 85% and 63%, respectively, according to the Myocardial Ischaemia National Audit Project (MINAP) admission data of our institute. Subsequently, the rate of progression to PCI was 91% and 71% for the STEMI and NSTEMI (or unstable angina) patients, respectively. Eighteen patients (6.1%) had advanced chronic kidney disease (estimated glomerular filtration rate [eGFR] <30 ml/min/1.73m2) and 49 patients (17%) had a history of previous coronary artery bypass graft (CABG) or PCI. Furthermore, transradial PCI was performed in 150 patients (51%). In addition, 282 patients (96%) had ≤2 vessels treated, 250 patients (85%) had ≤2 lesions treated and the number of patients receiving 1, 2 or ≥3 stents was 102 (34.7%), 82 (28%) and 72 (24.5%), respectively. Finally, 164 patients (55.8%) and 93 patients (31.6%) received bare metal stents (BMS) and drug-eluting stents (DES), respectively.
Clinical outcome

One-year follow-up was complete for all 294 patients. In-hospital, 30-day and one-year mortality rates were 2.4% (seven patients all presenting in cardiogenic shock), 4.4% (13 patients) and 17.7% (52 patients), respectively, for the entire cohort (table 2). Furthermore, in-hospital, 30-day and one-year mortality rates for the ACS and elective groups were 3.9% vs. 0% (p=0.033), 5.6% vs. 3.4% (p=0.24), and 20.0% vs. 14.0% (p=0.19), respectively. The in-hospital mortality rate for patients presenting with shock was 78%. In fact, there was zero mortality in the ACS group when the shock patients were excluded. The Kaplan–Meier curve representing one-year mortality rates in the ACS and elective groups is shown in figure 1. Furthermore, there was no significant statistical difference in one-year target vessel revascularisation (TVR) rates, which were 6.1% and 3.5% in the ACS and elective groups, respectively (p=0.33).

Subsequently, significant uni-variable predictors of one-year mortality were per year increase in age, male sex, shock and previous PCI. In fact, multi-variable analysis using Cox proportional hazard model revealed that the same parameters, i.e. age (HR 1.14, 95% CI 1.04 to 1.26), male sex (HR 1.86, 95% CI 1.01 to 3.43), previous PCI (HR 2.62, 95% CI 1.30 to 5.31) and the presence of shock (HR 15.81, 95% CI 6.86 to 36.50) emerged as independent predictors of one-year mortality rate as shown in table 3.

Discussion
To our knowledge, this paper represents the largest UK-based single-centre study examining the efficacy and safety of PCI in a large cohort of patients ≥85 years. Our data indicate that PCI is safe in patients ≥85 years with low in-hospital, 30-day and one-year mortality rates, irrespective of whether the PCI is performed in the setting of an ACS or electively. Patients ≥85 years presenting with cardiogenic shock recorded extremely high in-hospital mortality rates. In addition, per year incremental increase in age and being male increased the risk of one-year all-cause mortality by 14% and 85%, respectively.
Patients ≥85 years represent around 12% of all ACS admissions to UK hospitals according to a recent MINAP report.12 Furthermore, this cohort recorded the longest length of hospital stay and highest in-hospital mortality rate. Despite these observations, this age group was least likely to receive revascularisation, with only 10% of patients undergoing a diagnostic coronary angiogram. This age group, however, recorded the largest decrease in in-hospital mortality rates in comparison with patients <55 years (45% vs. 20%, respectively) over a seven-year period. We strongly agree with the observation of the authors of this report that “the elderly hospitalised with an ACS continue to be disadvantaged”.
The Global Registry of Acute Coronary Syndromes (GRACE) recently demonstrated that, following an ACS, octogenarians were less likely to receive standard secondary prevention treatment, in comparison with younger patients, despite recording the highest in-hospital mortality rate.13 The very elderly patients that underwent revascularisation during the index admission recorded the greatest absolute reduction in in-hospital death in comparison with other age groups. For example, the in-hospital mortality rates in patients <70 years old decreased from 2.9% to 1.6% following revascularisation in comparison with a decline from 11% to 7% in the very elderly. Subsequently, revascularisation in the very elderly translated to a 7% absolute reduction in all-cause mortality at six months as compared with a 1.8% reduction in the younger cohort. The in-hospital and six-month mortality rates in our study are comparable with those of the GRACE registry patients that received revascularisation, 3.9% vs. 7.0% and 11.7% vs. 12.0%, respectively.
Approximately, 39% of patients in our study underwent PCI for refractory stable angina, despite being on two or more anti-anginal agents. To date, no trial has examined the role of revascularisation in patients with stable angina ≥85 years old. The TIME trial was the first randomised trial to compare an invasive strategy versus medical therapy in 301 patients aged ≥75 years with stable angina.5 At six months, there was no difference in the all-cause mortality rate between the two groups. However, 50% of patients in the medical therapy group suffered an ACS and 75% of these underwent revascularisation. Subsequently, in a four-year follow-up of the TIME study, the sustained beneficial effect of revascularisation on the quality of life and angina status was noted.14 More importantly, revascularisation within one year of randomisation decreased the risk of long-term cardiac mortality by 56%. Finally, an invasive strategy was cost-effective in comparison with medical therapy.15
In concordance with our results, a large US-based registry demonstrated an in-hospital mortality rate of 5.4% in patients ≥85 years old, which was significantly higher in comparison with younger age groups.6 This age group recorded the most rapidly declining PCI rate over a four-year period. In contrast, patients ≥85 years old undergoing PCI represented the only group with a reduction in repeat revascularisation rates. The study also reported TVR rates of 7.0%, which are comparable with our data.6
Our data demonstrate that cardiogenic shock emerged as the strongest predictor of one-year all-cause mortality with an in-hospital mortality rate of 78%. These results are consistent with existing data.16 Furthermore, recent studies have also confirmed our findings of increasing age and male sex as independent predictors of one-year mortality rates.15,17 Previous PCI emerged as an independent predictor of one-year all-cause mortality in our study. Very elderly patients are at a greater risk of stent thrombosis.18 This is a consequence of several age-related biological changes, including endothelial dysfunction, higher levels of clotting factors and increased platelet reactivity.19 In the absence of any post-mortem data and relatively low event rates in our study it
was not possible to make this link.
Randomised trials have conventionally underrepresented the very elderly, comprising up to 2% of the study population.4 For example, the Clinical Outcomes Utilising Revascularisation and Aggressive Drug Evaluation (COURAGE) trial did not include any patients >75 years.20 In contrast, the proportion of patients ≥85 years in large registries has been five-fold higher.8 We feel that the evidence base to treat this age group can be strengthened substantially with future randomised-controlled trials.
Limitations
This is a single-centre retrospective study and is prone to inherent bias. Statistical tools were utilised to minimise this bias. Our findings are ‘hypothesis-generating’ and support the need for a randomised-controlled trial to examine the role, safety and efficacy of PCI in patients ≥85 years old. We were unable to ascertain the cognitive or frailty status for our cohort, which are important comorbidities in this age group and can influence management decisions and treatment options.
Conclusion
PCI is a safe and efficacious treatment option in very elderly patients with good one-year survival rates. There appears to be no difference in one-year survival rates between patients aged ≥85 years requiring acute or elective PCI. Future randomised-controlled trials should specifically include this age group to guide interventional cardiologists in making decisions when faced with this very challenging cohort.
Funding
This study was supported by the research fund of The Dorset Heart Centre, Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust.
Conflict of interest
None declared.
Editors’ note
See also the editorial by Knight et al.
Key messages
- There are very limited data examining the role of percutaneous coronary intervention (PCI) in the very elderly
(>85 years), who suffer from refractory angina or acute coronary syndrome (ACS) - Registry data suggest that the very elderly are less likely to receive PCI in comparison with younger patients and yet derive the greatest benefit in terms of survival and re-hospitalisation rates
- Our data indicate that PCI is a safe and acceptable treatment strategy for very elderly patients who present with ACS or refractory angina
- There is a need for randomised-controlled trials to examine the role of PCI in the very elderly
References
- National Population Projections 2010-based Statistical Bulletin. London: Office for National Statistics, 2011. Available from: http://www.ons.gov.uk/ons/dcp171778_235886.pdf
- Driver JA, Djoussé L, Logroscino G et al. Incidence of cardiovascular disease and cancer in advanced age: a prospective cohort study. BMJ 2008;337:a2467. http://dx.doi.org/10.1136/bmj.a2467
- Claessen BEPM, Kikkert WJ, Engstrom AE et al. Primary percutaneous coronary intervention for ST elevation myocardial infarction in octogenarians: trends and outcomes. Heart 2010;96:843–7. http://dx.doi.org/10.1136/hrt.2009.185678
- Alexander KP, Newby K, Cannon CP et al. Acute coronary care in the elderly, part 1 non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology in collaboration with the Society of Geriatric Cardiology. Circulation 2007;115:2549–69. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.182615
- Pfisterer M, Bertel O, Erne P et al. Trial of invasive versus medical therapy in elderly patients with chronic symptomatic coronary artery disease (TIME): a randomised trial. Lancet 2001;358:951–7. http://dx.doi.org/10.1016/S0140-6736(01)06100-1
- Wang TY, Masoudi FA, Messenger JC et al. Percutaneous coronary intervention and drug-eluting stent use among patients ≥85 years of age in the United States. J Am Coll Cardiol 2012;59:105–12. http://dx.doi.org/10.1016/j.jacc.2011.10.853
- Johnman C, Oldroyd KG, Mackay DF et al. Percutaneous coronary intervention in the elderly: changes in case-mix and periprocedural outcomes in 31,758 patients treated between 2000 and 2007. Circ Cardiovasc Interv 2010;3:341–5. http://dx.doi.org/10.1161/CIRCINTERVENTIONS.109.928705
- Alexander KP, Roe MT, Chen AY et al. Evolution in cardiovascular care for elderly patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE national quality improvement initiative. J Am Coll Cardiol 2005;46:1479–87. http://dx.doi.org/10.1016/j.jacc.2005.05.084
- Hochman JS, Sleeper LA, Webb JG et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med 1999;341:625–34. http://dx.doi.org/10.1056/NEJM199908263410901
- Van de Werf F, Bax J, Betriu A et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation. Eur Heart J 2008;29:2909–45. http://dx.doi.org/10.1093/eurheartj/ehn416
- Hamm CW, Bassand J-P, Agewall S et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2011;32:2999–3054. http://dx.doi.org/10.1093/eurheartj/ehr236
- Gale CP, Cattle BA, Woolston A et al. Resolving inequalities in care? Reduced mortality in the elderly after acute coronary syndromes. The Myocardial Ischaemia National Audit Project 2003–2010. Eur Heart J 2012;33:630–9. http://dx.doi.org/10.1093/eurheartj/ehr381
- Devlin G, Gore JM, Elliott J et al. Management and 6-month outcomes in elderly and very elderly patients with high-risk non-ST-elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Eur Heart J 2008;29:1275–82. http://dx.doi.org/10.1093/eurheartj/ehn124
- Pfisterer M. Long-term outcome in elderly patients with chronic angina managed invasively versus by optimized medical therapy: four year follow-up of the randomized trial of invasive versus medical therapy in elderly patients (TIME). Circulation 2004;110:1213–18. http://dx.doi.org/10.1161/01.CIR.0000140983.69571.BA
- Claude J, Schindler C, Kuster GM et al. Cost-effectiveness of invasive versus medical management of elderly patients with chronic symptomatic coronary artery disease: findings of the randomized trial of invasive versus medical therapy in elderly patients with chronic angina (TIME). Eur Heart J 2004;25:2195–203. http://dx.doi.org/10.1016/j.ehj.2004.09.013
- Zeymer U, Vogt A, Zahn R et al. Predictors of in-hospital mortality in 1333 patients with acute myocardial infarction complicated by cardiogenic shock treated with primary percutaneous coronary intervention (PCI). Eur Heart J 2004;25:322–8. http://dx.doi.org/10.1016/j.ehj.2003.12.008
- Roe MT, Chen AY, Thomas L et al. Predicting long-term mortality in older patients after non-ST-segment elevation myocardial infarction: the CRUSADE long-term mortality model and risk score. Am Heart J 2011;162:875–83. http://dx.doi.org/10.1016/j.ahj.2011.08.010
- Klein LW. Is patient frailty the unmeasured confounder that connects subacute stent thrombosis with increased periprocedural bleeding and increased mortality? J Am Coll Cardiol 2012;59:1760–2. http://dx.doi.org/10.1016/j.jacc.2012.01.042
- Wang TY, Gutierrez A, Peterson ED. Percutaneous coronary intervention in the elderly. Nat Rev Cardiol 2011;8:79–90. http://dx.doi.org/10.1038/nrcardio.2010.184
- Boden WE, O’Rourke RA, Teo KK. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16. http://dx.doi.org/10.1056/NEJMoa070829