An unusual case of endomyocardial fibrosis presenting secondary to idiopathic hypereosinophilic syndrome, diagnosed with the aid of cardiovascular magnetic resonance (CMR) imaging. This case highlights how CMR imaging is a powerful addition to current non-invasive diagnostic tools, for early clinical diagnosis of eosinophilic endomyocardial disease, and may potentially obviate the need for cardiac biopsy in the future.
Case report
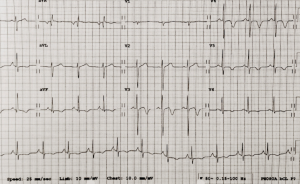
showing deep inferolateral T-wave inversion
A 43-year-old male presented to the Emergency Department with a five-day history of diarrhoea, vomiting, intermittent diplopia and headaches. Notably, there was no significant past medical history or history of travel to tropical or subtropical regions. He was a smoker without other cardiovascular risk factors. There was no family history of significant cardiac disorders.
On admission, the patient was haemodynamically stable and apyrexial (blood pressure [BP] 111/79 mmHg; heart rate [HR] 80 bpm; temperature 36.5°C). Clinical and, in particular, cardiovascular examination was unremarkable.
Routine hematological investigations revealed a raised eosinophil count of 12.4 × 109 cells/L (normal range 0.04–0.4 × 109 cells/L) and an elevated C-reactive protein of 39 mg/L (normal range 0–5 mg/L). A routine electrocardiogram (ECG) showed deep inferolateral T-wave inversion (figure 1).
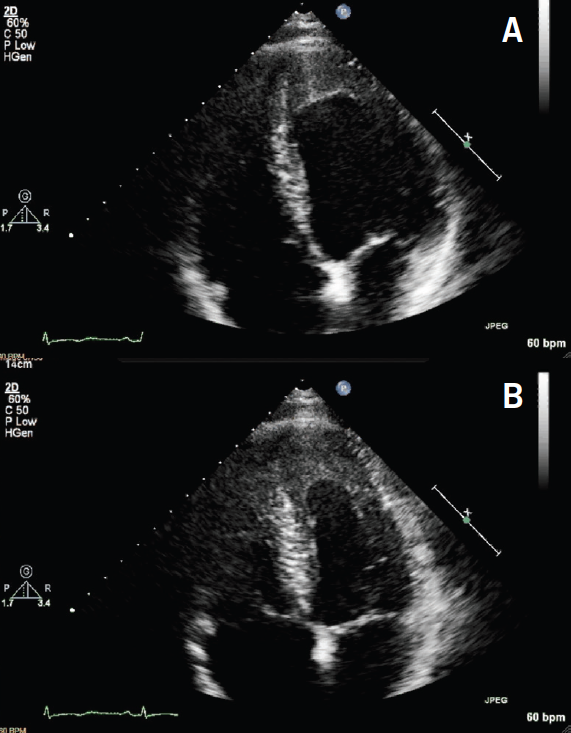
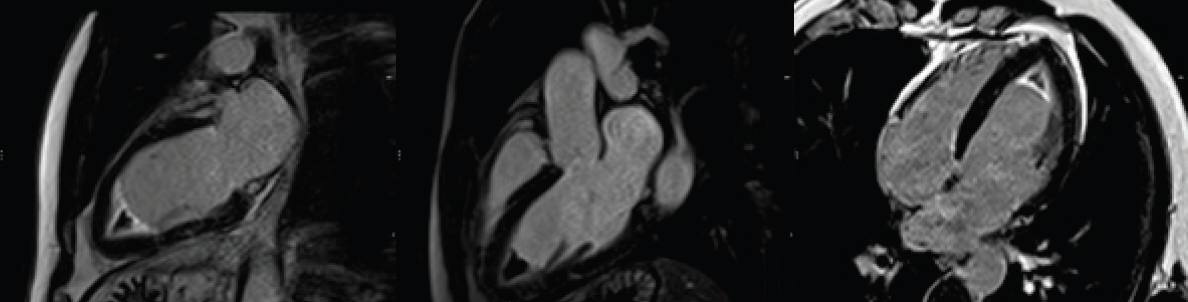
Additionally, cardiac biomarkers were elevated with troponin T of 1.7 ng/ml (abnormal >1 ng/ml). A transthoracic echocardiogram (TTE) was reassuring, demonstrating preserved left ventricular systolic function (ejection fraction [EF] >55%) with left ventricular apical hypertrophy (figure 2). Due to the abnormal ECG and elevated biomarkers, the initial differential diagnosis included a non-ST elevated myocardial infarction versus myocarditis versus apical hypertrophic cardiomyopathy. A coronary angiogram was performed, which showed normal coronaries with mild apical hypokinesis. Consequently, a cardiovascular magnetic resonance (CMR) scan was performed to look for a myocardial abnormality. This demonstrated preserved ventricular dimensions and function, however, late gadolinium images demonstrated apical subendocardial enhancement, suggesting the presence of endomyocardial fibrosis (EMF). Extensive endocardial thrombus was also noted (figures 3–5).
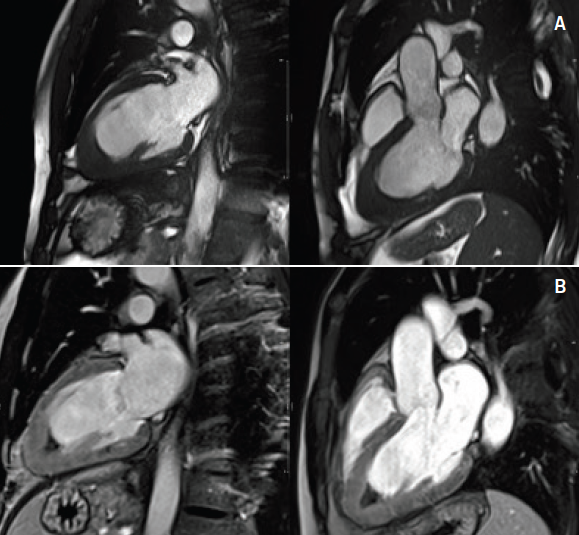
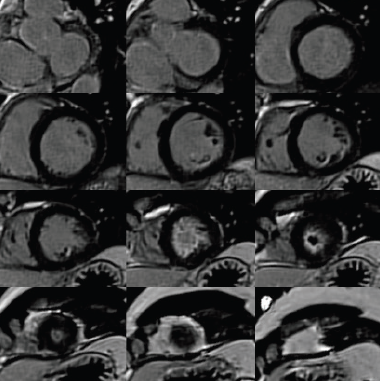
Given the haematological findings of hypereosinophilia and clinical evidence of cardiac involvement, a tentative diagnosis of idiopathic hypereosinophilic syndrome (HES) causing EMF was made. Referral was made to a haematological unit and specialist heart failure unit for further investigation and management; treatment with immunosuppressive therapy was commenced. Despite thorough investigation, no secondary cause of eosinophilia was determined.
Follow-up at six months revealed persistent eosinophilia of indeterminate cause. A final confirmed diagnosis of idiopathic HES causing EMF was, therefore, confirmed.
Discussion
Hypereosinophilic syndrome (HES) was first described in 1968 and is characterised by unexplained persistent eosinophilia with evidence of end organ damage.1,2 HES is associated with end organ damage to a wide spectrum of different systems, including cardiac, neurological, and gastrointestinal systems.3,4 Notably, cardiac involvement is particularly common, with 60–75% of patients with HES having severe heart failure.5
Cardiac involvement in HES often presents as endomyocarditis and may evolve into EMF as this case illustrates. EMF is characterised by the formation of fibrous tissue on the endocardium and myocardium of the inflow tract and apex of one or both ventricles with superimposed thrombus and calcification in advanced stages.6,7
The clinical findings vary depending on the location of the fibrosis. Right ventricular EMF may present with chronic systemic venous hypertension leading to elevated jugular pressure, gross hepatomegaly and congestive splenomegaly.8 Left ventricular EMF may present with a loud left ventricular third heart sound, short systolic murmur and severe pulmonary hypertension.9 Biventricular involvement presents with a combination of these findings.
Common ECG findings of EMF include P-wave abnormalities, reduced QRS voltages, and flattened or inverted T-waves.10 TTE was previously the primary imaging tool for identifying EMF. Typical TTE findings include endocardial thickening, fibro-thrombotic obliteration of the ventricular apices and valvular regurgitation due to the restricted motion of the posterior mitral leaflet.11
Endomyocardial biopsy was considered the gold standard to confirm the diagnosis of cardiac involvement in HES, however, the role of biopsy has been significantly reduced due to the ability of CMR imaging to accurately assess the extent of myocardial fibrosis throughout the entire myocardium. CMR imaging is, therefore, advocated for early diagnosis, intervention, and follow-up of patients with suspected cardiac involvement in HES.12,13
Early intervention in the management of EMF in HES is vital. If diagnosed early, at the time of myocarditis, treatment with immunosuppressive therapies may prevent disease progression.14,15 If the disease develops to a restrictive cardiomyopathy, the opportunity to prevent fibrosis recedes and management shifts to supportive measures. In advanced cases of EMF, surgical endocardial decortication may be considered as a palliative procedure.16 Symptoms of heart failure are treated with conventional cardiac medications.17 In all cases, prophylactic anticoagulation for thrombus prevention should be considered.
Outcomes of EMF in HES are poorly documented with limited contemporary data available. A Swiss group reported overall survival was 65% at five years and 59% at 10 years, with only marginal improvement in post-endocardial resection of EMF patients of various causes,18 however, fibrosis is known to recur even after surgery. Although debate still continues between Churg-Strauss syndrome (a condition with hypereosinophilia, allergic state and vasculitis) and HES, 10-year survival of EMF in the setting of Churg-Strauss has been reported.19 An experience reported by the Mayo Clinic recently on 247 HES patients only reported five cardiovascular-related deaths with one cardiomyopathy established, although no definite diagnosis of fibrosis was documented.20 Thus, survival of EMF is confounded by the cause of the fibrosis, the burden of the disease, and certainly the availability of supportive treatment available.
Conclusion
CMR imaging is an important and powerful addition to current non-invasive diagnostic tools for early clinical diagnosis of eosinophilic endomyocardial disease, and may potentially obviate the need for cardiac biopsy in the future.
Conflict of interest
None declared.
References
1. Hardy W, Anderson W. The hypereosinophilic syndromes. Ann Intern Med 1968;68:1220–9. http://dx.doi.org/10.7326/0003-4819-68-6-1220
2. Princess UO, Douglas RR, McDonald KH. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am 2007;27:457–75. http://dx.doi.org/10.1016/j.iac.2007.07.001
3. Chusid MJ, Dale DC, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore) 1975;54:1–27. http://dx.doi.org/10.1097/00005792-197501000-00001
4. Corseemit EP, Trip MD, Durrer JD. Löffler’s endomyocarditis in the idiopathic hypereosinophilic syndrome. Cardiology 1999;91:272–6. http://dx.doi.org/10.1159/000006923
5. Parrillo JE. Heart disease and the eosinophil. N Engl J Med 1990;323:1560–1. http://dx.doi.org/10.1056/NEJM199011293232211
6. Fawzy ME, Ziady G, Halim NM et al. Endomyocardial fibrosis: report of eight cases. J Am Coll Cardiol 1985;5:983–8. http://dx.doi.org/10.1016/S0735-1097(85)80444-7
7. Iglezias SA, Benvenuti LA, Calabrese F et al. Endomyocardial fibrosis: pathological and molecular findings of surgically resected ventricular endomyocardium. Virchows Arch 2008;453:233–41. http://dx.doi.org/10.1007/s00428-008-0652-3
8. Fernàndez VE, Lacarcel BC, Alcazar NB, Casado MI, Espejo GA, Ruiz CE. Chronic thromboembolic pulmonary hypertension associated with endomyocardial fibrosis of the right ventricle. Arch Bronconeumol 2003;39:370–2.
9. Vijayaraghavan G, Cherian G, Krishnaswami S, Sukumar IP. Left ventricular endomyocardial fibrosis in India. Br Heart J 1977;39:563–8. http://dx.doi.org/10.1136/hrt.39.5.563
10. Williams AW, Somers K. The electrocardiogram in endomyocardial fibrosis. Br Heart J 1960;22:311–15. http://dx.doi.org/10.1136/hrt.22.3.311
11. Kleinfeldt T, Nienaber CA, Kische S et al. Cardiac manifestation of the hypereosinophilic syndrome: new insights. Clin Res Cardiol 2010;99:419–27. http://dx.doi.org/10.1007/s00392-010-0144-8
12. Pillar N, Halkin A, Aviraz G. Hypereosinophilic syndrome with cardiac involvement: early diagnosis by cardiac magnetic resonance imaging. Can J Cardiol 2012;28:515.e11–e13. http://dx.doi.org/10.1016/j.cjca.2012.01.003
13. Looi JL, Ruygrok P, Royle G, Raos Z, Hood C, Kerr AJ. Acute eosinophilic endomyocarditis: early diagnosis and localization of the lesion by cardiac magnetic resonance imaging. Int J Cardiovasc Imaging 2010;26(suppl 1):151–4. http://dx.doi.org/10.1007/s10554-009-9580-9
14. Cortes J, Ault P, Koller C et al. Efficacy of imatinib mesylate in the treatment of idiopathic hypereosinophilic syndrome. Blood 2003;101:4714–16. http://dx.doi.org/10.1182/blood-2003-01-0081
15. Rothenberg ME, Klion AD, Roufosse FE et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med 2008;358:1215–28. http://dx.doi.org/10.1056/NEJMoa070812
16. Moraes F, Lapa C, Hazin S, Tenorio E, Gomes C, Moraes CR. Surgery for endomyocardial fibrosis revisited. Eur J Cardiothorac Surg 1999;15:309–12. http://dx.doi.org/10.1016/S1010-7940(99)00027-5
17. Hunt SA, Abraham WT, Chin MH et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 2005;112:154–235. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.167586
18. Schneider U, Jenni R, Turina J, Turina M, Hess OM. Long term follow up of patients with endomyocardial fibrosis: effects of surgery. Heart 1998;79:362–7.
19. McGavin CR, Marshall AJ, Lewis CT. Churg-Strauss syndrome with critical endomyocardial fibrosis: 10 year survival after combined surgical and medical management. Heart 2002;87:e5. http://dx.doi.org/10.1136/heart.87.5.e5
20. Podjasek JC, Butterfield JH. Mortality in hypereosinophilic syndrome: 19 years of experience at Mayo Clinic with a review of the literature. Leuk Res 2013;37:392–5. http://dx.doi.org/10.1016/j.leukres.2012.12.016