To determine whether the ‘smoker’s paradox’ exists in the acute coronary syndrome (ACS) population, we performed the first meta-analysis of adjusted risk estimates separately for early and late mortality. Eligible studies were comparative studies of smokers versus non-smokers enrolling patients hospitalised for ACS and reporting adjusted risk estimates for all-cause mortality.
Twenty-six risk-adjusted studies of smokers versus non-smokers enrolling >700,000 patients with ACS were identified and included. Pooled analysis suggested that smoking was associated with a statistically significant reduction in early (in-hospital or 30-day) mortality for the comparison of current versus never smokers (odds ratio [OR] 0.85; 95% confidence interval [CI] 0.75 to 0.96), any comparisons (current vs. never, former vs. never, current vs. former/never, and current/former vs. never smokers; OR 0.89; 95% CI 0.84 to 0.94), and patients with exclusive ST-segment elevation myocardial infarction (OR 0.80; 95% CI 0.73 to 0.87) and acute myocardial infarction (OR 0.87; 95% CI 0.82 to 0.92). Smoking was associated with a statistically non-significant increase in late mortality for any comparisons (hazard ratio 1.07; 95% CI 0.95 to 1.21).
In conclusion, the ‘smoker’s paradox’ for mortality may exist in the early phase following ACS but it may vanish in the late phase.
Introduction
Following observations that smokers experience decreased mortality following acute myocardial infarction (acute MI [AMI]) in comparison with non-smokers,1 the term ‘smoker’s paradox’ was introduced into scientific discourse more than 25 years ago.2 The ‘smoker’s paradox’ following various reperfusion strategies, however, is argued not to be due to any benefit from smoking itself but simply due to smokers being likely to undergo such procedures at a much younger age, and, hence, having, on average, lower comorbidity. In a recent systematic review (with a search by September 2010)2 of 17 studies presenting adjusted total mortality rates, the ‘smoker’s paradox’ was observed in some studies of AMI patients in the pre-thrombolytic and thrombolytic era, whereas no studies of a contemporary population with acute coronary syndrome (ACS) (including ST-segment elevation MI [STEMI], non-ST-segment elevation [NSTE] MI [NSTEMI], and unstable angina pectoris [UAP]) have found evidence for such a paradox. The authors, however, abstracted risk estimates for mortality in the longest follow-up from each individual study and, further, did not quantitatively combine them in meta-analytic methods. Although the reduced mortality was observed in smokers with AMI during their stay in the intensive care unit,3 smoking was an independent predictor of one-year mortality.4 Thus, it remains unclear whether the ‘smoker’s paradox’ exists, especially in the early phase in the ACS population. Adding more recent studies to the 17 studies included in the recent systematic review,2 we herein performed the first meta-analysis of adjusted risk estimates separately for early (in-hospital or 30-day) and late (>30-day) mortality in smokers (current or former) versus non-smokers (former or never) following ACS.
Methods
We used the same search strategy and study selection as the recent systematic review.2 To be brief, public domain databases including MEDLINE and EMBASE were searched through August 2012 using web-based search engines (PubMed and OVID). Studies considered for inclusion met the following criteria: the design was a comparative study of smokers versus non-smokers; the study population was patients hospitalised for ACS, including the previous World Health Organization (WHO) criteria for AMI5 and the more recent definition of ACS (including STEMI, NSTEMI and UAP);6 the study included >100 smokers and >100 non-smokers; and main outcomes included adjusted (minimum requirement as a covariate, ‘age’) risk estimates for all-cause mortality.
Data regarding detailed inclusion criteria, smoking type, duration of follow-up and all-cause mortality were abstracted (as available) from each individual study. For each study, early (in-hospital or 30-day) and late (>30-day) adjusted risk estimates for mortality, such as odds ratios (ORs), relative risks (RRs), hazard ratios (HRs), and their 95% confidence intervals (CIs), were extracted.
Study-specific estimates were combined using inverse variance-weighted averages of logarithmic risk estimates in the random-effects model. Between-study heterogeneity was analysed by means of standard χ2 tests. Four types of comparisons of smokers versus non-smokers were identified: current versus never smokers, former versus never smokers, current versus former/never smokers, and current/former versus never smokers. We selected the comparison of current versus never smokers as primary meta-analysis because of the strictest comparison. Secondary meta-analysis included any comparisons of smokers versus non-smokers: current versus never smokers (primary meta-analysis), former versus never smokers, current versus former/never smokers, and current/former versus never smokers. Sensitivity analyses were performed to assess the contribution of each study to the pooled estimate by excluding individual studies one at a time and recalculating the pooled risk estimates for the remaining studies. To assess the impact of differential diagnosis among studies on the pooled estimate, the effects of smoking on mortality were explored separately in studies enrolling patients with exclusive UAP, NSTEMI, NSTE ACS (including UAP and NSTEMI), STEMI, AMI, and ACS (including UAP, NSTEMI and STEMI). Publication bias was assessed graphically using a funnel plot and mathematically using adjusted rank-correlation and linear regression tests, according to the method of Begg and Mazumdar7 and Egger et al.8 All analyses were conducted using Review Manager version 5.1 (Nordic Cochrane Centre, Copenhagen, Denmark) and Comprehensive Meta-Analysis version 2 (Biostat, Englewood, NJ, USA).
Results
Our search identified 26 risk-adjusted studies3,4,9-32 of smokers versus non-smokers enrolling 700,341 patients with ACS (table 1). The dominant reperfusion strategy in most of these studies was thrombolysis (fibrinolysis). Only a few recent studies10,12,20 enrolled exclusive ACS patients undergoing primary percutaneous coronary intervention (PCI) including balloon angioplasty and stenting. The DANAMI-2 (Second Danish Multicenter Trial in Acute Myocardial Infarction)11 investigated whether an interaction between smoking status and the effect of primary PCI compared with fibrinolysis in patients with STEMI could be found. Smokers with short time to treatment (<3 hours) benefited equally from PCI and fibrinolysis with a trend toward higher mortality in the PCI group. In non-smokers with short time to treatment, PCI was superior to fibrinolysis for mortality. Patients with >3 hours to treatment all showed a tendency toward a superior effect of PCI irrespective of smoking habits. These findings suggest that smokers with STEMI and short time to treatment may benefit equally from fibrinolysis and PCI.
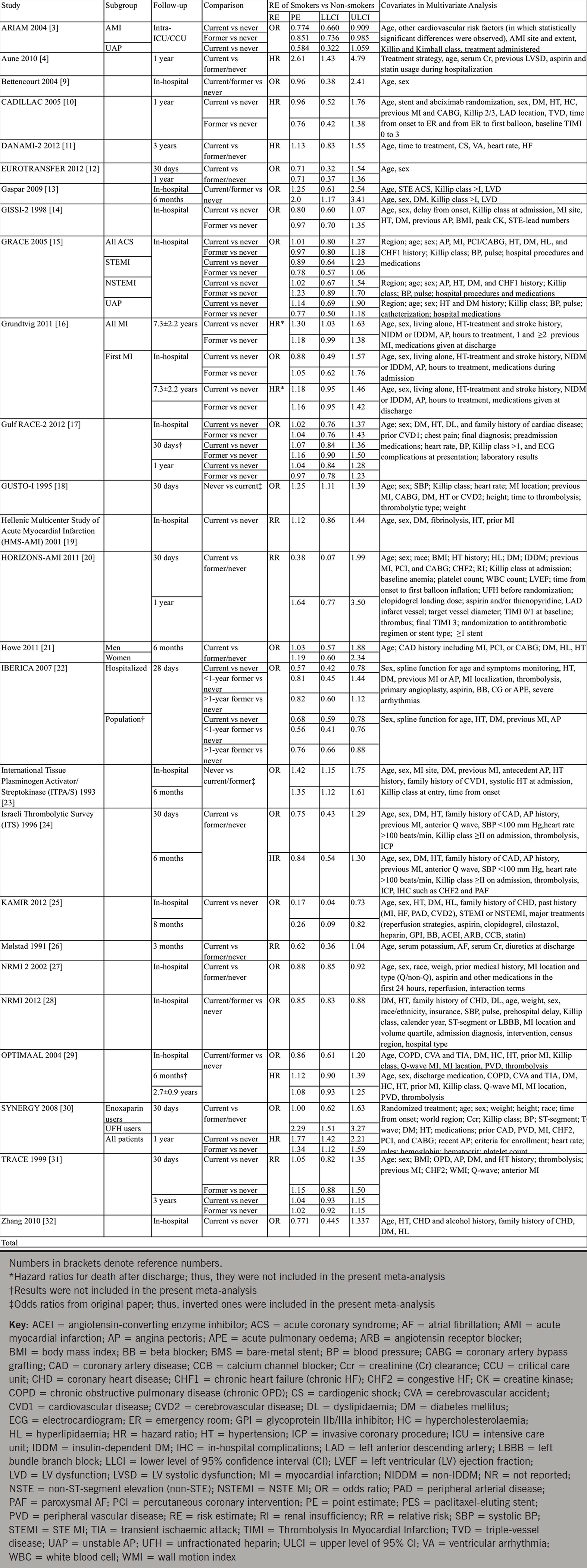
Table 1. Study characteristics, risk estimates for mortality, and covariates in multivariate analysis
View details
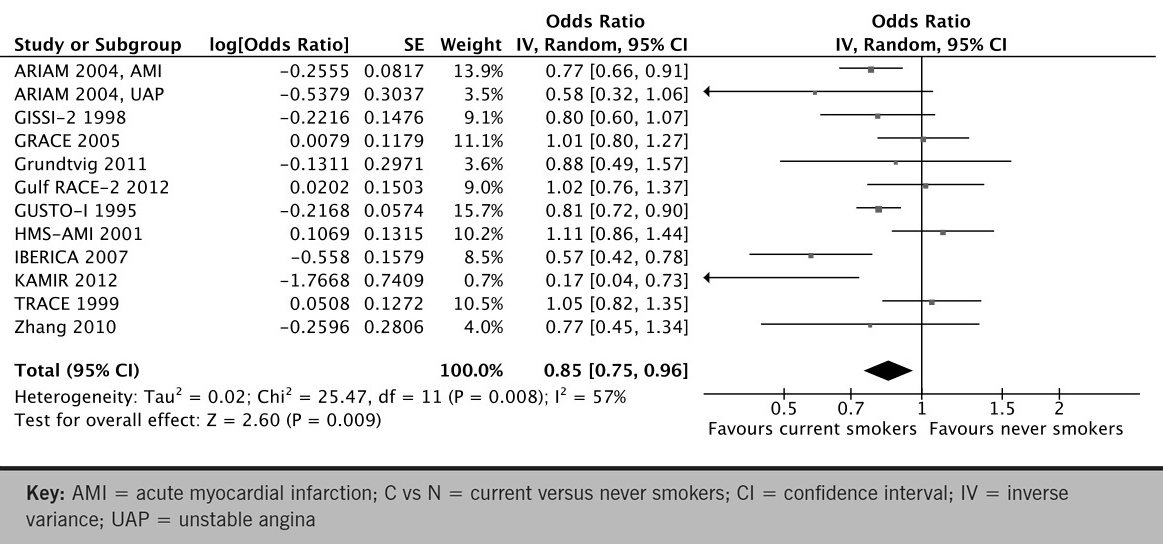
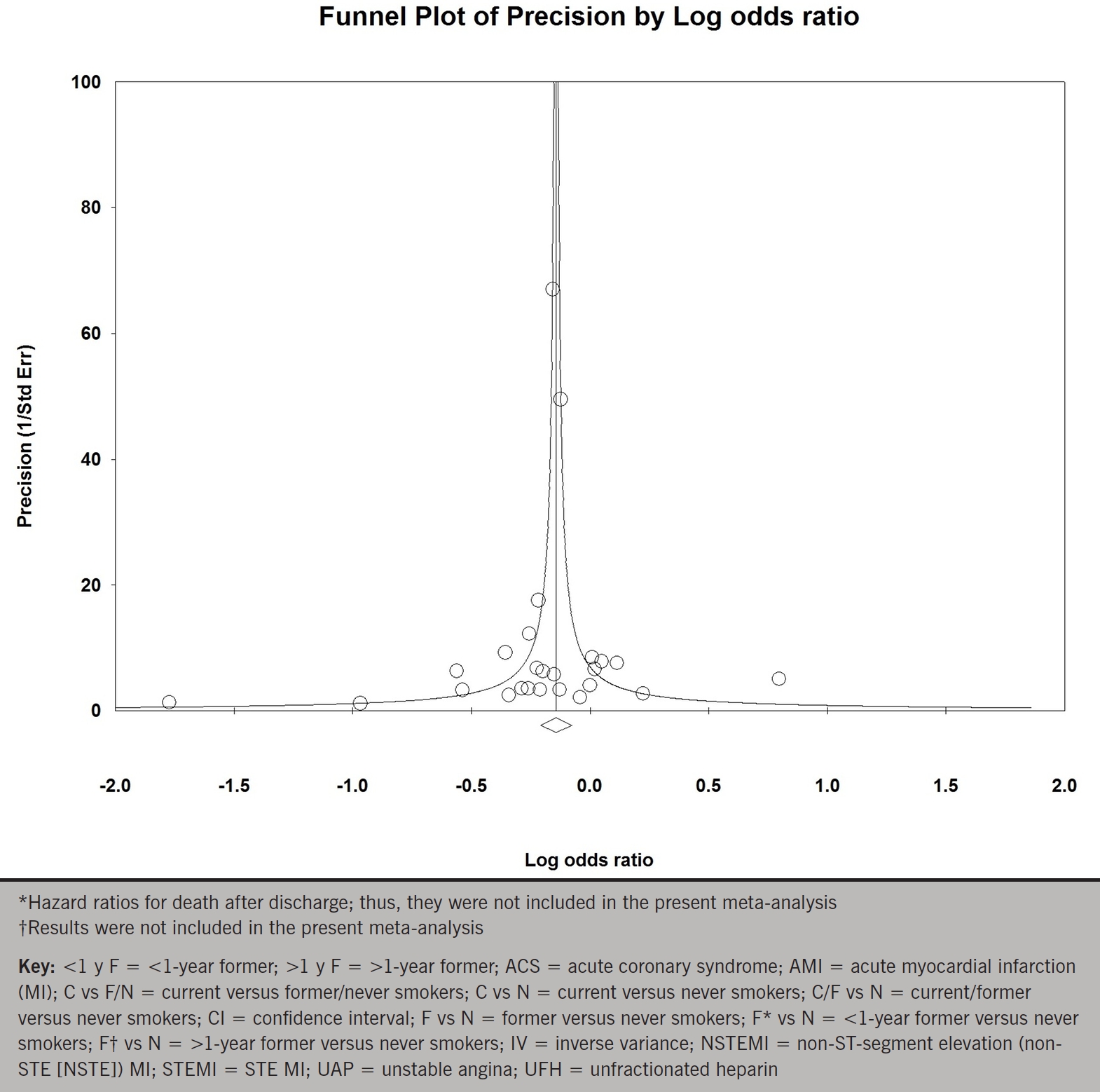
Pooled analysis of 11 studies of current versus never smokers (primary meta-analysis) demonstrated a statistically significant 15% reduction in early mortality with smokers relative to non-smokers (OR 0.85; 95% CI 0.75 to 0.96; p for effect =0.009; p for heterogeneity =0.008; figure 1A). Exclusion of any single study from the analysis did not substantively alter the overall result (figure 1B). To assess publication bias we generated a funnel plot of the logarithm of effect size versus the precision (reciprocal of standard error) for each study (figure 1C). There was no evidence of significant publication bias (p=0.30 and p=0.62 by Begg adjusted rank-correlation and Egger linear regression test, respectively).
Figure 1a. Forest plot of odds ratios for early mortality for the comparison of current versus never smokers
View details
Figure 1b. One-study-removed metaanalysis of early mortality for the comparison of current versus never smokers
View details
Figure 1c. Funnel plot of the logarithm of effect size for early mortality versus the precision (reciprocal of standard error) for each study of the comparison of current versus never smokers
View details
When data from 21 studies of any comparisons were pooled, smoking was associated with an 11% reduction in early mortality relative to non-smokers that remained statistically significant (OR 0.89; 95% CI 0.84 to 0.94; p for effect <0.0001; p for heterogeneity =0.0002; figure 2A). Eliminating any single study from the analysis did not substantially change the pooled estimate (figure 2B). There was no significant funnel plot asymmetry (p=0.43 and p=0.62; figure 2C). Pooled analysis of six studies enrolling patients with exclusive STEMI, and 10 studies enrolling patients with exclusive AMI, respectively, demonstrated a statistically significant 20% and 13% reduction in early mortality with smokers relative to non-smokers (OR for STEMI 0.80; 95% CI 0.73 to 0.87; p for effect <0.00001; p for heterogeneity =0.77; OR for AMI 0.87; 95% CI 0.82 to 0.92; p for effect <0.00001; p for heterogeneity =0.03; figure 2D).
Figure 2a. Forest plot of odds ratios for early mortality according to comparisons
View details
Figure 2b. One-study-removed meta-analysis of early mortality for any comparisons of smokers versus non-smokers
View details
Figure 2c. Funnel plot of the logarithm of effect size for early mortality versus the precision (reciprocal of standard error) for each study of any comparisons of smokers versus non-smokers
View details
Figure 2d. Forest plot of odds ratios for earlymortality according to events
View details
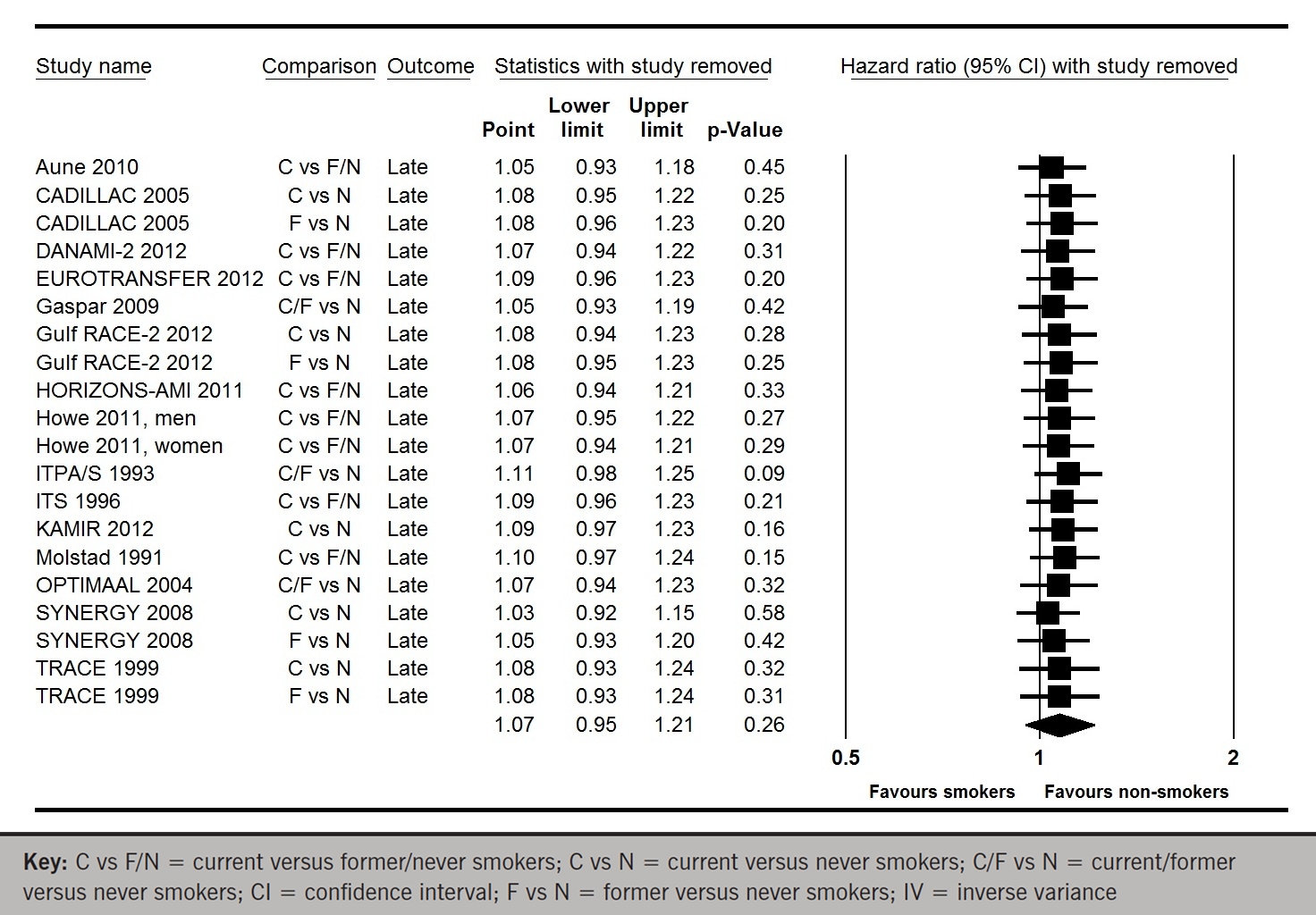
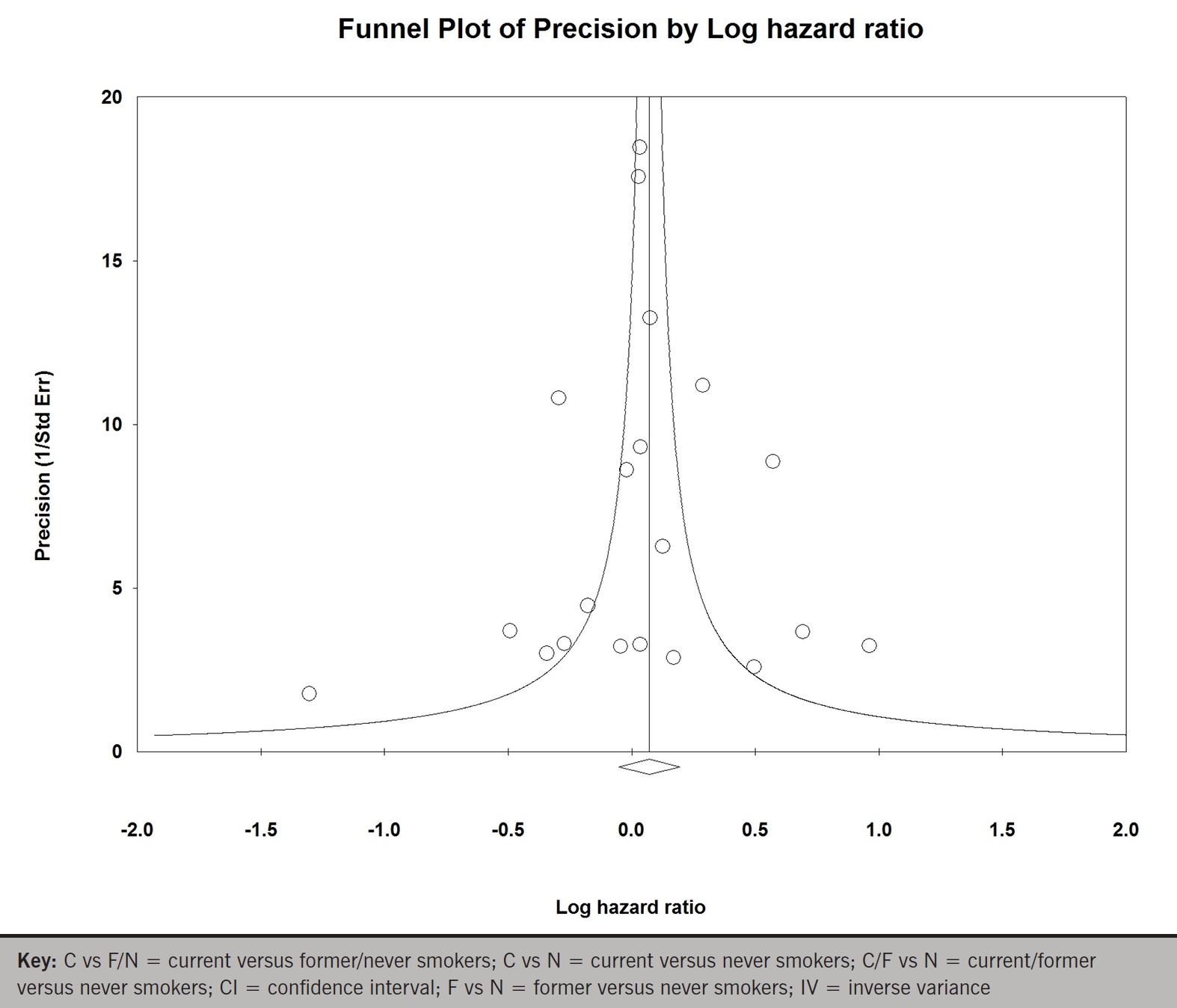
Whereas, when data from all 15 studies for late mortality were pooled, smoking was associated with a statistically non-significant 7% increase in mortality relative to non-smokers (HR 1.07; 95% CI 0.95 to 1.21; p for effect =0.26; p for heterogeneity <0.00001; figure 3A). Excluding any single study from the analysis did not substantively alter the summary measure (figure 3B). There was no significant publication bias (p=0.82 and p=0.98; figure 3C).
Figure 3a. Forest plot of hazard ratios for late mortality according to comparisons
View details
Figure 3b. One-study-removed meta-analysis of late mortality for any comparisons of smokers versus non-smokers
View details
Figure 3c. Funnel plot of the logarithm of effect size for late mortality versus the precision (reciprocal of standard error) for each study of any comparisons of smokers versus non-smokers
View details
Discussion
The results of our analysis suggest that the ‘smoker’s paradox’ for mortality may exist in the early phase (in-hospital or 30-day) following ACS, but it may vanish in the late phase (>30-day). The results were robust in sensitivity analyses, even eliminating any single study from the analysis. One possible mechanism for the paradoxical lower mortality in smokers might be a ‘pre-conditioning’-like effect of nicotine, an active component of the tobacco smoke. To test this hypothesis, it was investigated whether nicotine, given acutely before an ischaemic-reperfusion insult in a dose simulating blood levels of human smokers, attenuates MI in the rabbit.33 Nicotine given intravenously before ischaemic insult, however, did not protect the myocardium. Meanwhile, there may be slight differences in the pathogenesis of the acute coronary event in smokers as compared to non-smokers.2 Smokers have a relatively hypercoagulable state documented by increased haematocrit and fibrinogen levels, and quantitative coronary angiographic analysis suggests that the mechanism of infarction in smokers is more often thrombosis of a less critical atherosclerotic lesion compared with non-smokers.34 Further, smokers with STEMI improve myocardial perfusion after fibrinolytic therapy as compared with non-smokers.35 This finding lends support to the hypothesis that smokers have more complete clot lysis after fibrinolytic administration, which results in improved myocardial perfusion independent of epicardial artery flow. Smoking may also enhance circulating tissue factor activity and blood thrombogenicity.36 This suggests a more fibrin-rich thrombus in smokers with STEMI, which would leave them more amenable to fibrinolytic therapy and, thus, an improved survival rate.2
Other possible explanations for the reported paradoxical findings could be either due to systematic errors or residual confounding.2 Systematic errors would include publication bias. Our results may be influenced by a publication bias favouring smokers. This risk was minimised through an exhaustive search of the available literature. Though the statistical tests did not indicate publication bias, there is clearly limited power to detect such bias, given the small number of studies examined. Another systematic error might be that pre-hospital mortality could not be considered. Compared with non-smokers, smokers have a higher risk of dying before hospital admission, and the apparent decrease in case fatality in smokers after an acute cardiac event is restricted to patients who have been admitted.37 Thus, the ‘smoker’s paradox’ may be largely explained by a greater case fatality before admission to hospital in smokers. Adjustment for age and comorbidity reduced the magnitude of the smoking effect in many of the studies, but not all.2 The confounding variables, in addition to smoking status, used in the multi-variate analyses are presented in table 1, including a wide range of covariates for both baseline risk factors and provided treatment. On the one hand, a number of studies did not adjust for any treatment provided during hospitalisation; on the other hand, those included invasive treatment in the multi-variate analyses. Few studies included pulmonary disease29,31 and renal function4,26,30 in the multi-variate analyses. Part of the remaining effect could be due to residual confounding, both because of measurement errors in the cofactors and lack of information about relevant risk factors.2
Our analysis must be viewed in the context of its limitations. First, there was significant statistical between-study heterogeneity of risk estimates, e.g. p values of heterogeneity for early mortality were 0.008 and 0.0002 in the comparison of current versus never smokers (figure 1) and in any comparisons of smokers and non-smokers (figure 2), respectively. Heterogeneity, by which we mean variation among the results of individual studies beyond that expected from chance alone, is an important issue in meta-analysis. When heterogeneity is present, it may be inappropriate to combine the separate study estimates into a single number, particularly using fixed-effects methods that assume a common treatment effect.38 Random-effects methods, which provide an attractive approach to summarising heterogeneous results, model heterogeneity as variation of individual study treatment effects around a population average effect. Thus, we used not the fixed- but random-effects model to combine study-specific estimates.
Second, in all studies included in the present meta-analysis, the definition of smokers was dependent upon patients’, or their relatives’, personal statement without urine-cotinine measurement, which may misclassify true smokers into false non-smokers. Although non-smokers never declare themselves to be smokers, smokers are sometimes, or often likely, to declare themselves to be non-smokers. It is difficult, however, to evict such bias completely.
Third, in most of the studies included in the present meta-analysis, thrombolysis was predominantly performed as reperfusion therapy. A few studies published more recently suggest that ‘smoker’s paradox’ in early phase following ACS may not exist in the current primary-PCI era. In the KAMIR (Korea Acute Myocardial Infarction Registry) study published in 2012,25 1,218 young AMI patients (≤45 years of age) were enrolled. Primary PCI was performed in 73.3% of STEMI patients. Multi-variable logistic analysis showed that current smoking was an independent protective predictor of in-hospital total death. In subgroup analysis of patients undergoing PCI after STEMI, however, current smoking was not seen to be an independent protective factor for total death. In the HORIZONS-AMI (Harmonising Outcomes with Revascularisation and Stents in Acute Myocardial Infarction) trial published in 2011,20 3,602 STEMI patients were randomly assigned to unfractionated heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin alone and paclitaxel-eluting stents versus bare-metal stents. At 30 days, smokers had significantly lower rates of all-cause death compared with non-smokers. However, after multi-variable adjustment, the difference was no longer significant. Further, a total of 1,086 STEMI patients treated with primary PCI were enrolled into the EUROTRANSFER (European Registry on Patients with ST-Elevation MI Transferred for Mechanical Reperfusion with a Special Focus on Upstream Use of Abciximab) registry published 2012.12 Unadjusted mortality at 30 days was lower in current smokers compared with non-smokers. However, after adjustment for age and gender by logistic regression, there was no longer a significant difference between groups. Despite these findings, DANAMI-211 suggests that smokers with STEMI and short time to treatment may benefit equally from fibrinolysis and PCI. If so, the ‘smoker’s paradox’ would continue to exist even in the primary-PCI era.
Conclusion
Despite these acknowledged limitations, we found that, based on a meta-analysis of 26 risk-adjusted studies enrolling >700,000 patients, smoking is likely associated with lower early (in-hospital or 30-day) mortality following ACS. The ‘smoker’s paradox’, however, vanished in the late phase (>30-day) without leaving any trace.
“So she sat on, with closed eyes, and half believed herself in Wonderland, though she knew she had but to open them again and all would change to dull reality …”
Alice’s Adventures in Wonderland by Lewis Carroll
Conflict of interest
None declared.
Key messages
- We performed a meta-analysis of 26 risk-adjusted studies enrolling patients with acute coronary syndrome
- Smoking is associated with a statistically significant reduction in early mortality for the comparison of current versus never smokers, any comparisons (current vs. never, former vs. never, current vs. former/never, and current/former vs. never smokers), and patients with exclusive ST-segment elevation myocardial infarction and acute myocardial infarction
- Smoking is associated with a statistically non-significant increase in late mortality for any comparisons
References
1. Kelly TL, Gilpin E, Ahnve S, Henning H, Ross J Jr. Smoking status at the time of acute myocardial infarction and subsequent prognosis. Am Heart J 1985;110:535–41. http://dx.doi.org/10.1016/0002-8703(85)90071-7
2. Aune E, Røislien J, Mathisen M, Thelle DS, Otterstad JE. The “smoker’s paradox” in patients with acute coronary syndrome: a systematic review. BMC Med 2011;9:97. http://dx.doi.org/10.1186/1741-7015-9-97
3. Ruiz-Bailén M, de Hoyos EA, Reina-Toral A, Torres-Ruiz JM, Alvarez-Bueno M, Gómez Jiménez FJ; ARIAM Group. Paradoxical effect of smoking in the Spanish population with acute myocardial infarction or unstable angina: results of the ARIAM register. Chest 2004;125:831–40. http://dx.doi.org/10.1378/chest.125.3.831
4. Aune E, Endresen K, Roislien J, Hjelmesaeth J, Otterstad JE. The effect of tobacco smoking and treatment strategy on the one-year mortality of patients with acute non-ST-segment elevation myocardial infarction. BMC Cardiovasc Disord 2010;10:59. http://dx.doi.org/10.1186/1471-2261-10-59
5. Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation 1979;59:607–09. http://dx.doi.org/10.1161/01.CIR.59.3.607
6. Myocardial infarction redefined – a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J 2000;21:1502–13. http://dx.doi.org/10.1053/euhj.2000.2305
7. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. http://dx.doi.org/10.2307/2533446
8. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. http://dx.doi.org/10.1136/bmj.315.7109.629
9. Bettencourt N, Mateus P, Dias C et al. The smoker’s – a hemodynamic reality? Rev Port Cardiol 2004;23:547–55.
10. Weisz G, Cox DA, Garcia E et al. Impact of smoking status on outcomes of primary coronary intervention for acute myocardial infarction – the smoker’s paradox revisited. Am Heart J 2005;150:358–64. http://dx.doi.org/10.1016/j.ahj.2004.01.032
11. Rasmussen T, Kelbæk H, Madsen JK et al. Smokers with ST-segment elevation myocardial infarction and short time to treatment have equal effects of PCI and fibrinolysis. J Invasive Cardiol 2012;24:401–06.
12. Rakowski T, Siudak Z, Dziewierz A, Dubiel JS, Dudek D. Impact of smoking status on outcome in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Thromb Thrombolysis 2012;34:397–403. http://dx.doi.org/10.1007/s11239-012-0764-0
13. Gaspar A, Nabalis S, Rocha S et al. Smoking in acute coronary syndromes – the “smoker’s paradox” revisited. Rev Port Cardiol 2009;28:425–37.
14. Maggioni AP, Piantadosi F, Tognoni G, Santoro E, Franzosi MG. Smoking is not a protective factor for patients with acute myocardial infarction: the viewpoint of the GISSI-2 Study. G Ital Cardiol 1998;28:970–8.
15. Himbert D, Klutman M, Steg G, White K, Gulba DC; GRACE Investigators. Cigarette smoking and acute coronary syndromes: a multinational observational study. Int J Cardiol 2005;100:109–17. http://dx.doi.org/10.1016/j.ijcard.2004.10.004
16. Grundtvig M, Hagen TP, Amrud ES, Reikvam A. Mortality after myocardial infarction: impact of gender and smoking status. Eur J Epidemiol 2011;26:385–93. http://dx.doi.org/10.1007/s10654-011-9557-6
17. Al Suwaidi J, Al Habib K, Singh R et al. Tobacco modalities used and outcome in patients with acute coronary syndrome: an observational report. Postgrad Med J 2012;88:566–74. http://dx.doi.org/10.1136/postgradmedj-2011-130178
18. Barbash GI, Reiner J, White HD et al. Evaluation of paradoxic beneficial effects of smoking in patients receiving thrombolytic therapy for acute myocardial infarction: mechanism of the “smoker’s paradox” from the GUSTO-I trial, with angiographic insights. Global Utilization of Streptokinase and Tissue-Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol 1995;26:1222–9. http://dx.doi.org/10.1016/0735-1097(95)00299-5
19. Andrikopoulos GK, Richter DJ, Dilaveris PE et al. In-hospital mortality of habitual cigarette smokers after acute myocardial infarction; the “smoker’s paradox” in a countrywide study. Eur Heart J 2001;22:776–84. http://dx.doi.org/10.1053/euhj.2000.2315
20. Goto K, Nikolsky E, Lansky AJ et al. Impact of smoking on outcomes of patients with ST-segment elevation myocardial infarction (from the HORIZONS-AMI trial). Am J Cardiol 2011;108:1387–94. http://dx.doi.org/10.1016/j.amjcard.2011.06.063
21. Howe M, Leidal A, Montgomery D, Jackson E. Role of cigarette smoking and gender in acute coronary syndrome events. Am J Cardiol 2011;108:1382–6. http://dx.doi.org/10.1016/j.amjcard.2011.06.059
22. Elosua R, Vega G, Rohlfs I et al.; IBERICA investigators. Smoking and myocardial infarction case-fatality: hospital and population approach. Eur J Cardiovasc Prev Rehabil 2007;14:561–7. http://dx.doi.org/10.1097/HJR.0b013e32804955b3
23. Barbash GI, White HD, Modan M et al. Significance of smoking in patients receiving thrombolytic therapy for acute myocardial infarction. Experience gleaned from the International Tissue Plasminogen Activator/Streptokinase Mortality Trial. Circulation 1993;87:53–8. http://dx.doi.org/10.1161/01.CIR.87.1.53
24. Gottlieb S, Boyko V, Zahger D et al. Smoking and prognosis after acute myocardial infarction in the thrombolytic era (Israeli Thrombolytic National Survey). J Am Coll Cardiol 1996;28:1506–13. http://dx.doi.org/10.1016/S0735-1097(96)00334-8
25. Chen KY, Rha SW, Li YJ et al.; for the Korea Acute Myocardial Infarction Registry Investigators. ‘Smoker’s paradox’ in young patients with acute myocardial infarction. Clin Exp Pharmacol Physiol 2012;39:630–5. http://dx.doi.org/10.1111/j.1440-1681.2012.05721.x
26. Mølstad P. First myocardial infarction in smokers. Eur Heart J 1991;12:753–9. http://dx.doi.org/10.1093/eurheartj/12.7.753
27. Gourlay SG, Rundle AC, Barron HV. Smoking and mortality following acute myocardial infarction: results from the National Registry of Myocardial Infarction 2 (NRMI 2). Nicotine Tob Res 2002;4:101–07. http://dx.doi.org/10.1080/14622200110103205
28. Canto JG, Kiefe CI, Rogers WJ et al.; NRMI Investigators. Atherosclerotic risk factors and their association with hospital mortality among patients with first myocardial infarction (from the National Registry of Myocardial Infarction). Am J Cardiol 2012;110:1256–61. http://dx.doi.org/10.1016/j.amjcard.2012.06.025
29. Jaatun HJ, Sutradhar SC, Dickstein K; OPTIMAAL Study Group. Comparison of mortality rates after acute myocardial infarction in smokers versus nonsmokers. Am J Cardiol 2004;94:632–6, A9.
30. Mahaffey KW, Yang Q, Pieper KS et al.; SYNERGY Trial Investigators. Prediction of one-year survival in high-risk patients with acute coronary syndromes: results from the SYNERGY trial. J Gen Intern Med 2008;23:310–16. http://dx.doi.org/10.1007/s11606-007-0498-4
31. Jørgensen S, Køber L, Ottesen MM, Torp-Pedersen C, Videbaek J, Kjøller E. The prognostic importance of smoking status at the time of acute myocardial infarction in 6676 patients. TRACE Study Group. J Cardiovasc Risk 1999;6:23–7.
32. Zhang H, Sun S, Tong L et al. Effect of cigarette smoking on clinical outcomes of hospitalized Chinese male smokers with acute myocardial infarction. Chin Med J (Engl) 2010;123:2807–11. http://dx.doi.org/10.3760/cma.j.issn.0366-6999.2010.20.011
33. Birnbaum Y, Hale SL, Kloner RA. Is preconditioning by nicotine responsible for the better prognosi in smokers with acute myocardial infarction? Basic Res Cardiol 1996;91:240–7.
34. Grines CL, Topol EJ, O’Neill WW et al. Effect of cigarette smoking on outcome after thrombolytic therapy for myocardial infarction. Circulation 1995;91:298–303. http://dx.doi.org/10.1161/01.CIR.91.2.298
35. Kirtane AJ, Martinezclark P, Rahman AM et al. Association of smoking with improved myocardial perfusion and the angiographic characterization of myocardial tissue perfusion after fibrinolytic therapy for ST-segment elevation myocardial infarction. J Am Coll Cardiol 2005;45:321–3. http://dx.doi.org/10.1016/j.jacc.2004.10.018
36. Sambola A, Osende J, Hathcock J et al. Role of risk factors in the modulation of tissue factor activity and blood thrombogenicity. Circulation 2003;107:973–7. http://dx.doi.org/10.1161/01.CIR.0000050621.67499.7D
37. Sonke GS, Stewart AW, Beaglehole R, Jackson R, White HD. Comparison of case fatality in smokers and non-smokers after acute cardiac event. BMJ 1997;315:992–3. http://dx.doi.org/10.1136/bmj.315.7114.992
38. Engels EA, Schmid CH, Terrin N, Olkin I, Lau J. Heterogeneity and statistical significance in meta-analysis: an empirical study of 125 meta-analyses. Stat Med 2000;19:1707–28. http://dx.doi.org/10.1002/1097-0258(20000715)19:13<1707::AID-SIM491>3.0.CO;2-P