Providing cardiopulmonary bypass and surgical back-up for transcatheter aortic valve implantation has significant implications for surgical services. It is unclear how practice varies around the UK and whether valve-type influences practice. We performed an email-based survey to gain a UK-wide snapshot of current practice. We found that bypass was available in the catheter lab in 94% of Edwards versus 30% of CoreValve centres (p=0.0003), and that a full surgical team and theatre were kept free in 89% of Edwards versus 20% of CoreValve centres (p=0.008). Further research is required to understand whether this difference in surgical provision, related to valve-type, confers outcome benefit.
Introduction
The provision of cardiopulmonary bypass (CPB) equipment and cardiothoracic (CT) surgical back-up during transcatheter aortic valve implantation (TAVI) has major implications for surgical services. Consensus statements from the Society of Cardiothoracic Surgery (SCTS) and the British Cardiovascular Intervention Society (BCIS) recommend that centres performing TAVI should have “immediate availability of perfusion services in case of the need for emergency bypass”, and that this, together with other criteria, mean that TAVI should only be performed in units currently performing surgical aortic valve replacement. There has, however, been wide variation across the UK in the way in which these guidelines have been interpreted. Therefore, we aimed to:
1. Gather an observation of current UK practice with regards to CPB and surgical provision during TAVI.
2. Establish whether practice differed between centres implanting different valves.
Results
The TAVI lead (provided by the UK TAVI Steering Group) for all 33 UK TAVI centres was contacted via a questionnaire-based survey, comprising the following questions:
1. During a TAVI procedure is your CPB equipment:
a) In the catheter laboratory where the TAVI is being performed?
b) Reserved for use for the TAVI procedure, but in cardiac theatres?
c) Available as for usual PCI practice?
2. On the day of TAVI do you have a complete surgical team (surgeons, nurses, perfusionists, etc.) and a theatre free to convert to an open chest procedure in the event of a complication?
a) No, never.
b) For surgical access cases such as direct aortic, transapical or subclavian only.
c) For every case.
3. Which transcatheter heart valve (THV) do you predominantly implant (Edwards, CoreValve or both)?
Responses were obtained from all 33 centres; 18 (55%) centres implanted Edwards valves with 10 (30%) implanting CoreValve (Medtronic) and five (15%) implanting both. Answers to question 1 (figure 1) revealed that CPB equipment was available in the catheter lab in 22 (67%) centres (17 Edwards, three CoreValve, two both), reserved in cardiac theatres in a further five (15%) centres (one Edwards, three CoreValve, one both) and ‘informally’ available as per usual percutaneous coronary intervention (PCI) practice in the remaining six (18%) centres (0 Edwards, four CoreValve, two both, p=0.001). CPB equipment is present in the catheter lab during TAVI in virtually all (17/18, 94%) Edwards centres, as compared with 3/10 (30%) CoreValve centres (p=0.0003).
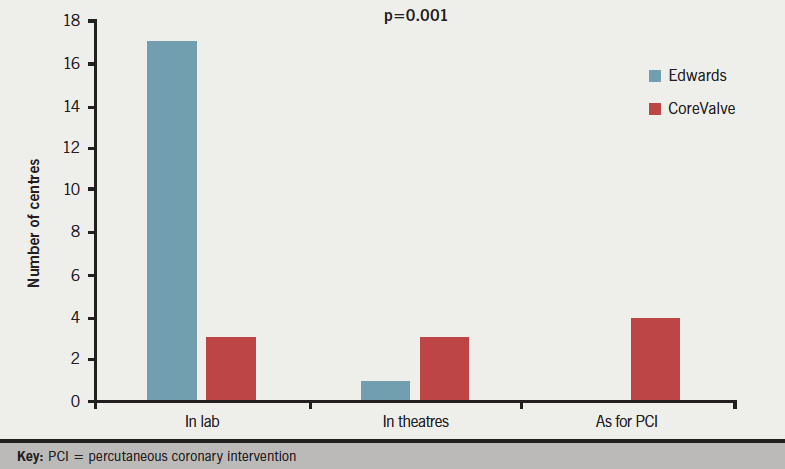
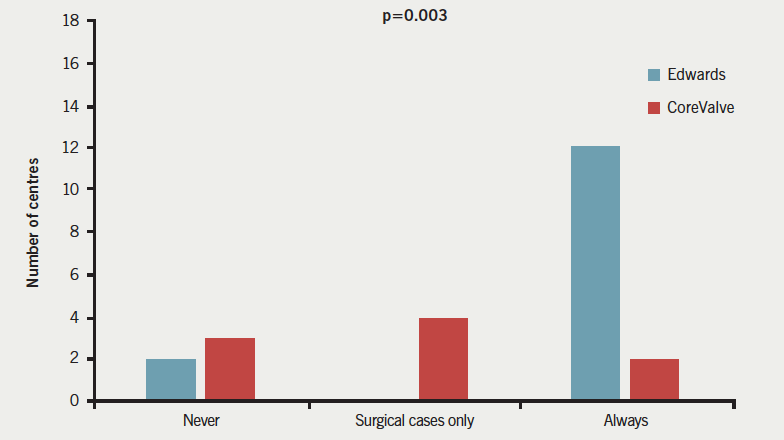
Responses to question 2 (figure 2) demonstrated that a complete theatre team and free theatre were available for all TAVI implants in 20 (52%) centres (16 Edwards, two CoreValve, two both), with six (18%) centres (0 Edwards, four CoreValve, two both) providing full surgical provision just for surgical cases, and a further six (18%) centres (two Edwards, three CoreValve, one both) never having a full surgical team and theatre on standby (p=0.003). The majority of Edwards implanting centres 16/18 (89%) had a surgical team and theatre free for all cases, as compared with just 2/10 (20%) of CoreValve centres (p=0.008).
Conclusion
Clear differences exist between centres, which are associated with the type of THV implanted. More centres using the Edwards valve had CPB available in the catheter lab and an empty theatre on standby. This difference in practice is likely explained by the differing origins of the devices: the CoreValve from an interventional device company and the Edwards valve from a surgical valve company. However, despite this difference the 30-day mortality rates following TAVI are similar in UK centres implanting Edwards and CoreValve prosthetic valves.1 Data were not gathered on whether the procedures were performed in a hybrid theatre, however, at the time of the questionnaire only a handful of centres had access to hybrid rooms. Having CPB available in the catheter lab, and a vacant CT theatre during a case, has significant resource implications and potential loss of income to the centre. The need for emergency surgical involvement is infrequent and its potential impact on outcomes is unlikely to be observed in a relatively small group of patients. These data are observational, and further research is required to explore whether these differences in availability of immediate CPB and surgery have an influence on outcomes in TAVI.
Author contributions
JS helped to design the questionnaire, analyse responses, wrote the manuscript and created the figures. VV assisted with analysing responses and manuscript review. JNT assisted with construction of the questionnaire, analysis of responses and amending the manuscript. PFL was responsible for assisting the design of the questionnaire, performed all email contact with the UK centres, received all responses and helped analyse responses and the writing of the manuscript (corresponding author). SND assisted with construction of the questionnaire, analysis of responses, writing and amendment of the manuscript.
Conflict of interest
None declared.
Editors’ note
See also the editorial on this topic by Christopher Allen et al. in this issue.
Key messages
- Immediate availability of cardiopulmonary bypass, theatres and surgical teams during transcatheter aortic valve implantation (TAVI) differs between centres using Edwards as compared with the CoreValve transcatheter heart valve
- Whether this difference influences outcomes, however, is unknown, and further research is required
Reference
1. Moat NE, Ludman P, de Belder MA et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis; the UK TAVI registry. J Am Coll Cardiol 2011;58:2130–8. http://dx.doi.org/10.1016/j.jacc.2011.08.050
