This study aimed to assess mortality and cardiovascular (CV) outcomes of patients with newly diagnosed atrial fibrillation (AF) managed in the UK primary care setting. Electronic patient records in The Health Improvement Network were used to identify incident AF (n=9,418, 52.1% male, mean age 73.8 years [standard deviation 11.2]) and matched (gender, age and locality) controls (n=47,090) aged ≥40 years. Three main study outcomes were assessed within two years of follow-up: incident CV outcomes, CV mortality and all-cause mortality. AF cases had an increased risk of developing all investigated CV outcomes when compared with controls (systemic hypertension relative risk [RR]=1.9 [95% confidence interval 1.7–2.1]; peripheral thromboembolic events RR=2.0 [1.8–2.4]; congestive heart failure RR=13.1 [11.5–14.8]; valvular heart disease RR=7.0 [6.0–8.1]; ischaemic heart disease RR=4.3 [3.8–4.8]; stroke RR=3.7 [3.3–4.2]; myocardial infarction RR=3.1 [2.6–3.6]). AF patients were also twice (RR=2.0 [1.8–2.1]) as likely to die from all causes and almost three times (RR=2.7 [2.4–3.1]) more likely to die from CV reasons than controls. AF cases demonstrated consistently worse prognosis across all of the main outcomes assessed when compared with the control patients.
Introduction
 Atrial fibrillation (AF) is a supraventricular tachyarrhythmia characterised by uncoordinated activation of the atria. AF is a progressive disease and represents the most common serious disorder of cardiac rhythm. The incidence and prevalence of the disease increase progressively with age and is more common among men.1–5
Atrial fibrillation (AF) is a supraventricular tachyarrhythmia characterised by uncoordinated activation of the atria. AF is a progressive disease and represents the most common serious disorder of cardiac rhythm. The incidence and prevalence of the disease increase progressively with age and is more common among men.1–5
AF is associated with higher mortality and cardiovascular (CV) morbidity.6–13 Specifically, AF is a recognised risk factor for stroke, with the proportion of strokes attributable to AF increasing exponentially with age.1,2,7,14–17 Although clinicians are most concerned about stroke risk among AF patients, congestive heart failure (CHF) is the most common outcome of AF.7,17,18
In 2006, guidelines were developed for the diagnosis and management of AF.19,20 These guidelines outline AF treatment and management including prevention of thromboembolism after AF diagnosis. Prevention of other CV outcomes, such as CHF, were included in later guidelines published in 2010.21 In the UK, the primary care general practitioner (GP) remains the most important healthcare professional in the management of long-term medical conditions, such as AF. The outcomes from AF are dependent upon the treatment choices made by these GPs and optimal management of AF can only be implemented if the natural history and outcomes of the disease are fully appreciated. This study aims to assess mortality and CV outcomes in a population of patients with newly diagnosed AF managed in the UK primary care setting.
Materials and methods
Data source
The data for this study came from The Health Improvement Network (THIN) database. At the time of this study, THIN contained primary care medical records on 5.6% of the overall population of the UK and it is generally considered representative of the general UK population.22–24 Patient-level medical records in THIN include demographic and comprehensive clinical information, such as diagnoses and symptoms, prescriptions, diagnostic tests, referrals and hospitalisation, and other health-relevant data. The study obtained ethical approval from the Cambridgeshire 4 Research Ethics Committee (REC) on 13 November 2009 (REC reference number 10/H0305/44).
Study population
The electronic medical records of patients in THIN practices were used to identify both the newly diagnosed AF cases and controls between May 2006 and November 2007.Patients were required to be 40 years or older. Patients receiving treatment for cancer or who were being actively followed-up for a cancer diagnosis (presence of cancer-specific medication or diagnosis code within the 18 months prior to study initiation [index date]) were excluded. In addition, patients were excluded if they had a record of acute thyrotoxicosis, intercurrent infection, or alcohol intoxication, within the 30 days before (and including) the index date. All cases and controls were required to be permanently registered at the general practice for a minimum of six months before index date.
Case patients
Patients with a first diagnosis of AF (based on Read Codes: G573.00, G573000, G573200, G573300, G573z00, G573400, G573500) during the recruitment period (1 May 2006 to 30 November 2007) were selected as case patients. The index date of cases was the date of entry of the first ever record of an AF diagnosis code. There were 9,418 cases identified that met the inclusion and exclusion criteria.
Control patients
Controls, randomly selected from the non-AF population, were matched 5:1 to their respective cases according to the following parameters: gender, age (±3 years) and location of practice (locality). The index date for controls was the same date as the index date of their respective matched cases. Potential controls with a record of receiving sotalol, amiodarone or flecainide prescriptions prior to index date were excluded. There were 47,090 controls who met the inclusion and exclusion criteria.
Follow-up
All patients were followed from the index date until the earliest date resulting in the end of two years’ follow-up or the end of the patient record (i.e. death, transferred out of the practice or end of data collection at the practice).
Outcomes and covariates
The three main outcomes of interest were all-cause mortality, CV mortality and incidence of CV events. CV-related events and mortality included AF, systemic hypertension, peripheral thromboembolic events (PTEE), CHF, valvular heart disease (VHD), ischaemic heart disease (IHD), myocardial infarction (MI) and stroke or transient ischaemic attack (TIA). Additional outcomes were hospitalisations and diagnoses of diabetes, depression, anxiety and panic disorders.
Data were also collected for the following covariates: age at index date; gender; Townsend quintile score of social deprivation (last score in the record – scores range from 1 to 5 with 1 being the most affluent); CHA2DS2-VASc score,26 hospitalisation for CV events; medical procedures including cardioversion and ablation.
The comorbid conditions, pharmacological therapy, procedures and hospitalisations were classified in two ways: 1) history at any time was defined as an indication in their record at any time prior to index date; and 2) recent history defined as an indication in their record at any time within one year prior to index.
Hospital episodes were defined because multiple record entries may take place in THIN for the same hospital event. Hospitalisations entered within 30 days of the first hospital record were assumed related to the same hospitalisation episode. Hospitalisations information around the index date was not included in counting the number of subsequent CV or AF admissions because hospitalisations within 30 days of the index date may not be due to a new CV or AF event but rather due to an admission related to the AF diagnosis. Specific diagnoses related to hospitalisations were not available and, hence, these analyses were not performed.
Death ascertainment
Mortality was assessed over the two years of follow-up plus an additional four months to allow for retrospective administrative recording of death. The cause of death was investigated for the 5,428 patients. Information on cause of death was sourced from coded information in THIN, free-text information in THIN or, if still unknown, death certificates when possible. Once collected, the cause of death was assigned as CV or ‘other’ by a minimum of two blinded reviewers. It was not possible to ascertain cause of death for 1,990 patients (530 cases [36.3% of cases who died] and 1,460 controls [36.9% of controls who died]). These patients were included in the all-cause mortality analysis but excluded from the CV mortality analysis.
Data analysis
Patient characteristics were summarised for the cases and controls. Differences in characteristics were examined with t-tests for the continuous variables and chi-squared tests for the categorical variables.
For each study outcome, the incidence rate was estimated as the number of patients with the outcome by the end of follow-up over the total person-years at risk. Incidence rates were calculated from patients without a history of the condition (ever recorded prior to index). For example, patients with a history of CHF were not included in the calculation of the incidence of CHF. Relative risks (RRs) controlling for age, gender and locality were calculated to compare the incidence rate of each outcome between the cases and controls.
Kaplan-Meier curves were created to assess time to the main study outcomes. Conditional logistic regression models were developed to estimate odds ratios (ORs) comparing risk of death for cases compared with controls while adjusting for important differences in patient characteristics.
Data analysis was performed in SAS v9.2 and SAS Enterprise v4.2.
Results
Table 1 displays the baseline characteristics of the 9,418 AF cases (52.1% male, mean age 73.8 years [standard deviation 11.2]) and 47,090 controls (52.1% male, mean age 73.5 years [11.2]) at the index date. As expected, there are no meaningful differences between the cases and controls for age and gender because these criteria were used to create matched pairs for the two populations. The two groups were also similar in distribution of social deprivation as measured by the Townsend score. There was statistical evidence (p≤0.001) to suggest a difference between cases and controls for a history of CHA2DS2-VASc, CV-related hospitalisations, cardioversion and ablation procedures.
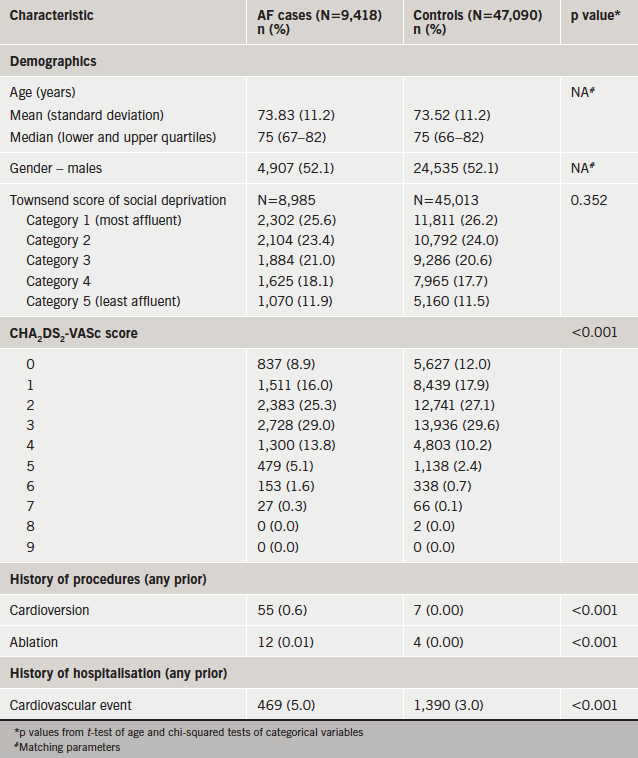
Over the follow-up time period, there was evidence to suggest differences in incidence rates between cases and controls for all outcomes measured controlling for age, gender and locality (table 2). RRs for several conditions were notably high with RRs above 3.0 including CHF (RR=13.1, 95% confidence interval [CI] 11.5–14.8), VHD (RR=7.0, 95% CI 6.0–8.1), IHD (RR=4.3, 95% CI 3.8–4.8), stroke and TIA (RR=3.7, 95% CI 3.3–4.2), MI (RR=3.1, 95% CI 2.6–3.6). In addition, cases were at greater than three times higher risk for hospitalisation for CV event compared with controls (RR=3.2, 95% CI 2.5–4.0).
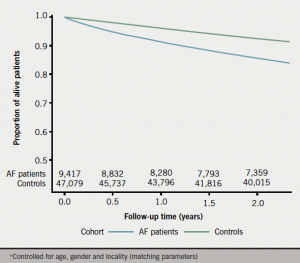
AF cases were more likely to die compared with controls of both all-cause mortality (RR=2.0, 95% CI 1.8–2.1) and CV-related mortality (RR=2.7, 95% CI 2.4–3.1) controlling for age, gender and locality (table 2). Similar results were found using conditional logistic regression controlling for age, gender, locality, Townsend score, CHA2DS2-VASc, valvular disease, and anticoagulant therapy (AF vs. controls all-cause mortality OR=2.3, 95% CI 2.1–2.5; CV mortality OR=2.8, 95% CI 2.4–3.3) (table 3). The Kaplan-Meier curves in figures 1 and 2 illustrate the differences in all-cause and CV-related mortality between cases and controls.


Discussion
This is one of the largest population-based studies of AF in the general practice setting. Newly diagnosed AF patients demonstrated consistently worse prognosis across a variety of outcomes compared with those without the diagnosis. These outcomes included CV-related hospitalisations, incidence of new CV outcomes (systemic hypertension, PTEE, CHF, VHD, IHD, MI, stroke or TIA), diabetes and mental health (anxiety and depression) diagnoses, and mortality (all-cause and CV).
Risk of developing other CV conditions
AF has been shown to be associated with increased risk of other major CV events including stroke and CHF.17,18,25 This study also showed the greatest risk for CHF (RR=13.1), VHD (RR=7.0), IHD (RR=4.3), stroke (RR=3.7) and MI (RR=3.1) when comparing cases with controls controlling for age, gender and locality. The RR for stroke, CHF and coronary events are comparable with those reported by Ruigomez et al.17 (ischaemic cerebrovascular event RR=3.0; coronary event RR=2.1; CHF RR=6.4) in a study utilising similar methodology.
To our knowledge, most studies focus on CV outcomes such as stroke and CHF, and treatment guidelines emphasise stroke prevention.19,20 Along with the major CV outcomes, this study also examined the risk of developing additional outcomes, such as diabetes (RR=2.1), anxiety and panic disorders (RR=1.8), depression (RR=1.8), systemic hypertension (RR=1.9) and PTEE (RR=2.0). These results would require further study, but imply that, beyond stroke, a broader CV, diabetes and mental health disease prevention programme should be evaluated.
Mortality
In this study, AF patients were at about a two-fold higher risk of CV mortality (OR=2.7) and all-cause mortality (OR=2.0) compared with controls. Other studies in a variety of countries have reported similar increased risk in CV mortality and the overall increased mortality among AF patients was related to the severity of underlying CV disease.6–8,12,25 In this study, the ORs were similar when a history of valvular disease, CHA2DS2-VASc scores and anticoagulant therapy was controlled for in multi-variable modelling (all-cause mortality OR=2.3; CV mortality OR=2.8). This would imply that AF patients are at increased risk of all-cause and CV mortality and appropriate prevention measures should be evaluated.
Limitations
The primary weakness of this study is that the diagnoses of AF, as well as the primary study outcomes, were not validated by examination or confirmatory test results. It cannot be ruled out that presentation with AF was the factor that precipitated investigation for and diagnosis of underlying CV disease in these patients. The study used information on AF cases that sought care by their GP and this would likely bias the results to include symptomatic cases. The data source used for the analysis has been shown to be representative of UK general practice.23,24
Another limitation is that cause of death could not be determined for 36% of the deaths. This means that the CV cause of death analysis should be interpreted in light of this potential bias. However, the missing causes of death were not differentially distributed between the AF patients (36% missing cause of death) and the controls (37% missing cause of death) so we do not anticipate that the mortality rates would change dramatically. In addition, our estimates are similar to those previously reported for CV mortality.6–8,12,25 This would not be an issue for the all-cause mortality analysis, since the cause of death was not required for this analysis.
Strengths
This is one of the largest population-based, case-control studies of AF with a total of 9,418 patients newly diagnosed with AF and 47,090 matched controls. The patient population included in the THIN database has been shown to be representative of the UK patient population with respect to gender and age distribution for the prevalence of major disease.24 Specifically, the crude prevalence of AF in THIN is comparable with that found in the UK.23,24
Conclusion
This study provides valuable, observational, population-based data to assess morbidity and mortality associated with AF. Other studies have shown that AF imposes considerable morbidity including hospitalisation and mortality.8,20,25 Given that we report a significant increased risk (RR ranging from 1.9 to 7.0) of new cardiovascular diagnoses (including systemic hypertension, PTEE, VHD, IHD, MI and diabetes), in addition to stroke and CHF, further investigation of prevention for these outcomes among AF patients is warranted. Despite the fact that AF patients are frequently referred to secondary or tertiary care centres for specialist advice and treatment in the UK, the majority of long-term disease management occurs in the general practice setting. Optimal disease management in this setting can be obtained by understanding the natural history and likely outcomes of AF in the general practice setting.
Acknowledgements
Mary Thompson: Senior Research Manager, Cegedim Strategic Data Medical Research Ltd.
Delphine Vial: Clinical Project Manager, Cegedim Strategic Data Medical Research Ltd.
Claire Cockbain: Clinical Project Leader, Sanofi UK.
Source of support
This study was fully funded by Sanofi UK, Guildford.
Conflict of interest
None declared.
Key messages
- This is one of the largest, observational, population-based studies of atrial fibrillation (AF) in the general practice setting
- Newly diagnosed AF patients demonstrated increases in the incidence of new cardiovascular diagnoses including stroke, congestive heart failure, myocardial infarction, ischaemic heart disease, valvular heart disease, peripheral thromboembolic events and systemic hypertension compared with individuals without AF
- Newly diagnosed AF patients had a two-fold increased risk of all-cause mortality and were 2.7 times more likely to experience CV mortality compared with those without the diagnosis
- Prevention of a variety of CV outcomes beyond stroke and congestive heart failure among AF patients should be examined further
References
1. Wolf PA, Abbott RD, Kannell WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–8. http://dx.doi.org/10.1161/01.STR.22.8.983
2. Rich MW. Epidemiology of atrial fibrillation. J Interv Card Electrophysiol 2009;25:3–8. http://dx.doi.org/10.1007/s10840-008-9337-8
3. Miyaska Y, Barnes ME, Bailey KR et al. Mortality trends in patients diagnosed with first atrial fibrillation. J Am Coll Cardiol 2007;49:986–92. http://dx.doi.org/10.1016/j.jacc.2006.10.062
4. Ruigomez A, Johansson S, Wallander M et al. Incidence of chronic atrial fibrillation in general practice and its treatment pattern. J Clin Epidemiol 2002;55:358–63. http://dx.doi.org/10.1016/S0895-4356(01)00478-4
5. Rietbrock S, Heely E, Plumb J et al. Chronic atrial fibrillation: incidence prevalence, and prediction of stroke using the congestive heart failure, hypertension, age > 75, diabetes mellitus, and prior stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J 2008;156:57–64. http://dx.doi.org/10.1016/j.ahj.2008.03.010
6. Benjamin EJ, Wolf PH, D’Agostino RB et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–52. http://dx.doi.org/10.1161/01.CIR.98.10.946
7. Krahn AD, Manfreda J, Tate RB et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-up Study. Am J Med 1995;98:476–84. http://dx.doi.org/10.1016/S0002-9343(99)80348-9
8. Miyaska Y, Barnes ME, Gersh BJ et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota 1980–2000, and implication on the projections for future prevalence. Circulation 2006;114:119–25. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.595140
9. Probst P, Goldschlanger N, Selzer A. Left atrial size and atrial fibrillation in mitral stenosis: factors influencing their relationship. Circulation 1973;48:1282–7. http://dx.doi.org/10.1161/01.CIR.48.6.1282
10. Stevenson WG, Stevenson LW, Middlekauff HR et al. Improving survival for patients with atrial fibrillation and advanced heart failure. J Am Coll Cardiol 1996;28:1458–63. http://dx.doi.org/10.1016/S0735-1097(96)00358-0
11. Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol 2003;91:2D–8D. http://dx.doi.org/10.1016/S0002-9149(02)03373-8
12. Ruigomez A, Johansson S, Wallander MA, Garcia Rodriguez LA. Risk of mortality in a cohort of patients newly diagnosed with chronic atrial fibrillation. BMC Cardiovasc Disord 2002;2:5. http://dx.doi.org/10.1186/1471-2261-2-5
13. Nieuwlaat R, Prins MH, Le Heuzey JY et al. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fibrillation. Eur Heart J 2008;29:1181–9. http://dx.doi.org/10.1093/eurheartj/ehn139
14. Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 2003;22:118–23. http://dx.doi.org/10.1159/000068743
15. Motoki H, Tomita T, Aizawa K et al. Coagulation activity is increased in the left atria in patients with paroxysmal atrial fibrillation during the pre-paroxysmal period: comparison with chronic atrial fibrillation. Circ J 2009;73:1403–07. http://dx.doi.org/10.1253/circj.CJ-09-0008
16. Ruigomez A, Garcia Rodriguez LA, Johansson S et al. Risk of cerebrovascular accident after a first diagnosis of atrial fibrillation. Clin Cardiol 2007;30:624–8. http://dx.doi.org/10.1002/clc.20178
17. Ruigomez A, Johansson S, Wallander M et al. Risk of cardiovascular and cerebrovascular events after atrial fibrillation diagnosis. Int J Cardiol 2009;136:186–92. http://dx.doi.org/10.1016/j.ijcard.2008.04.050
18. Tsang TSM, Miyaska Y, Barnes M et al. Epidemiological profile of atrial fibrillation: a contemporary perspective. Prog Cardiovasc Dis 2005;48:1–8. http://dx.doi.org/10.1016/j.pcad.2005.06.001
19. National Institute for Health and Care Excellence. The management of Atrial Fibrillation – Quick Reference Guide. London: NICE, 2006. Available from: http://guidance.nice.org.uk/CG36/QuickRefGuide/pdf/English
20. Fuster V, Rydén LE, Cannom DS et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006;114:e257–e354. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.177292
21. The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Guidelines for the management of atrial fibrillation. Eur Heart J 2010;31:2369–429. http://dx.doi.org/10.1093/eurheartj/ehq278
22. Hippisley-Cox, Coupland C, Vinogradova Y et al. Performance of the QRISK cardiovascular risk prediction algorithm in an independent UK sample of patients from general practice: a validation study. Heart 2008;94:34–9. http://dx.doi.org/10.1136/hrt.2007.134890
23. Blak BT, Thompson M. How does The Health Improvement Network (THIN) data on prevalences on chronic diseases compare with national figures? Value Health 2009;12:A253. http://dx.doi.org/10.1016/S1098-3015(10)74240-6
24. Blak BT, Thompson M, Dattani H et al. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011;19:251–5. Available from: http://www.ingentaconnect.com/content/bcs/ipc/2011/00000019/00000004/art00009?token=004e1b317eeb0b420f3275c277b42572b67287d66342444557645592f653b2a2d3a7c4e724770e
25. Charlemagne A, Blacher J, Cohen A et al. Epidemiology of atrial fibrillation in France: extrapolation of international epidemiological data to France and analysis of French hospitalization data. Arch Cardiovasc Dis 2011;104:115–24. http://dx.doi.org/10.1016/j.acvd.2010.11.012
26. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euroheart survey on atrial fibrillation. Chest 2010;137:263–72. http://dx.doi.org/10.1378/chest.09-1584