When the NOACs (novel oral anticoagulants) were introduced over three years ago, they promised to revitalise the management of conditions such as atrial fibrillation (AF), venous thromboembolism (VTE) and thromboprophylaxis after major joint replacement surgery. Rivaroxaban is currently available in multiple indications, including (but not limited to): prevention of stroke and systemic embolism in adult patients with non-valvular AF, treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and the prevention of recurrent DVT and PE in adults.
 For decades anticoagulant therapy in these conditions had relied on the vitamin K antagonists (VKAs; primarily warfarin) and low molecular weight heparin (LMWH). Both had shown themselves to be effective therapies, but with significant pharmacological and practical disadvantages and significant drug interactions affecting much of the population who go on to VKA therapy.
For decades anticoagulant therapy in these conditions had relied on the vitamin K antagonists (VKAs; primarily warfarin) and low molecular weight heparin (LMWH). Both had shown themselves to be effective therapies, but with significant pharmacological and practical disadvantages and significant drug interactions affecting much of the population who go on to VKA therapy.
The use of warfarin, for instance, is limited by its narrow therapeutic window, unpredictable pharmacokinetics, propensity to interact with a variety of foods and medications, as well as the need for individualised dosing and regular blood monitoring.1
Despite undoubted efficacy – reducing the risk of stroke by 68% (adjusted relative risk [ARR] 3.1%) in patients with AF2 – warfarin is used in only 55% of eligible patients.3
It has also been shown that only about half of all warfarin patients, regardless of indication, are within an effective therapeutic range.4
In contrast, the NOACs apixaban▼, rivaroxaban▼ and dabigatran offered the prospect of an oral therapy with a predictable therapeutic response, rapid onset of action, few drug interactions and no requirement for regular coagulation monitoring. Successful clinical trials were duly followed by regulatory approval and the stage seemed set for the NOACs to gradually usurp their more unwieldy predecessors. In the UK, however, the adoption of NOACs into frontline clinical practice has been surprisingly slow.
A number of reasons have been suggested for this; perhaps doctors are reluctant to lose the reassurance of blood monitoring and fear their patients may not comply with treatment without it. Unfamiliarity with dosing regimens, the current lack of an antidote and concerns over drug costs may also play a role.1 There may also be reservations as to whether ‘real-world’ practice is truly reflected in the sanitised world of the clinical trial.
It was with this in mind that a recent round-table meeting was held in London. Featuring a selection of clinical trialists, secondary care specialists and general practitioners (see list of participants above), the meeting focused on the practicalities of anticoagulant therapy in today’s healthcare system.
Real-life data versus clinical trials
The backdrop to the meeting was the release of data from the Dresden NOAC registry.5 This is a strictly observational registry of now more than 2,500 patients who have been prescribed NOACs under normal, clinical conditions. Most of the patients have been prescribed rivaroxaban either for stroke prevention in atrial fibrillation (SPAF) or for DVT or PE treatment.
In contrast to a clinical trial, the patients in the Dresden NOAC registry are selected by treating physicians with no inclusion or exclusion criteria, no strict protocols, no study-related interference to improve compliance and no study-specific interventions. The meeting participants were asked to consider whether such ‘real-life’ data are more relevant to the practising physician than results from clinical trials and, therefore, more likely to affect day-to-day practice. As might be expected among a panel featuring both front-line practitioners and clinical trial specialists, opinion was divided on the issue.
Some felt registry data offered valuable real-life information, suggesting that new non-interventional studies involving a wider spectrum of patients, selected by treating physicians, would complement the current clinical trial data.
Nevertheless, the characteristics of treated patients included in the Dresden registry do suggest that a different population is being studied than in the two major phase III clinical trials of rivaroxaban, ROCKET AF (Rivaroxaban versus Warfarin in Non-valvular Atrial Fibrillation) and EINSTEIN DVT and PE.
For example, the VTE patients in the registry are much older and with worse renal function than in the EINSTEIN trial. The SPAF patients are less likely to have had prior VKAs and have lower CHADS2 (cardiac failure, hypertension, age, diabetes, stroke) scores than those in the ROCKET AF trial.5-7
Risk of major bleeding
In the recent phase III NOAC trials in SPAF, patients treated with NOACs have previously been shown to have a similar risk of major bleeding as those treated with warfarin,which was around 3% per year.8 However, it is known that bleeding rates rise to around 7% per year in daily care settings, as has been demonstrated by several VKA registries.9
In addition to the high rates of VKA bleeding observed in daily care, patients suffering from major VKA-related bleeding have poor outcomes (table 1). Historical data, recorded in the same hospitals as those involved in the treatment of bleeding complications seen in the current Dresden cohort, show NOACs provide an opportunity for an indirect comparison of outcomes after VKA- and NOAC-associated bleeding complications.10
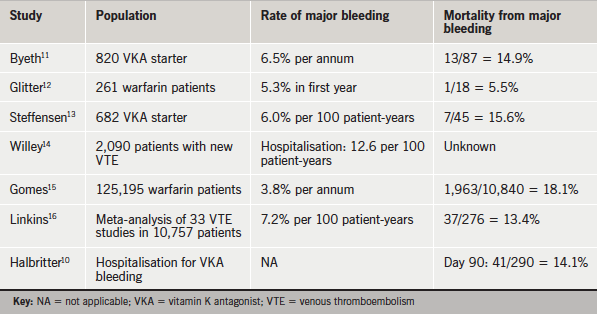
The meeting considered historical data collected by 22 hospitals in the larger area of Dresden throughout 2005, on all patients hospitalised for VKA-related bleeding. During this time:
- There were 290 hospitalisations for VKA-related bleeding
- The median hospital stay was 11 days
- Patients’ median international normalised ratio (INR) was 3.0 (within the target range)
- The in-hospital mortality was 7.6%
- Mortality at 90 days was 14.1%
- 15.3% of survivors were help-dependent.
 Contrasting data from the current Dresden NOAC registry indicate that patients hospitalised for rivaroxaban-associated bleedings in the same hospitals had better outcomes; there was a significant drop in both in-hospital bleeding-related mortality and 90-day mortality for bleeding-related hospitalisations.5
Contrasting data from the current Dresden NOAC registry indicate that patients hospitalised for rivaroxaban-associated bleedings in the same hospitals had better outcomes; there was a significant drop in both in-hospital bleeding-related mortality and 90-day mortality for bleeding-related hospitalisations.5
There was some debate among meeting participants over the validity of this comparison. Some felt that the historical data were bound to show inferior results, as management of oral anticoagulation has progressed significantly since 2005.
Others felt that any selection bias was unlikely to be in favour of the NOACs, as the first patients to be switched from VKAs to NOACs would be those who were not performing well on warfarin. The data were described as a graphic demonstration of the ‘catastrophic’ consequences of bleeding on VKAs.
In the pre-NOAC era, VKAs led to one in eight patients, who were hospitalised as a result of a bleed, dying after three months and even those who survived 90 days were likely to have significant impairment to their quality of life in terms of recurrent hospitalisations, recurrent bleeding episodes and potential surgical interventions.10
Consistent to the findings of the observational Dresden registries, a subgroup analysis from the ROCKET-AF trial demonstrated a better outcome after major bleeding in the NOAC arm in a head-to-head comparison of patients experiencing major bleeding complications during rivaroxaban or VKA treatment in this large phase III trial.6
The outcome of major bleeds resulting from rivaroxaban was found to be far more positive (critical bleed rate 1.3 vs. 1.9, hazard ratio (HR) 0.69, 95% confidence interval (CI) (0.53–0.91), p=0.007; and fatal bleed rate 0.4 vs. 0.8, HR 0.50, 95% CI (0.31–0.79), p=0.003) for patients on rivaroxaban and VKA, respectively, despite the inconsistent availability of emergency laboratory tests in all hospitals and the current lack of a licensed antidote to NOAC treatment.
Adherence: once-a-day versus twice-a-day dosing
Patient adherence on anticoagulation therapy, as with any medication, can be challenging; the panel discussed patient preference between once- or twice-daily dosing regimens on NOAC therapy, both in a trial setting and in clinical practice, and considered the extent to which this may affect compliance.
Participants agreed that while there was little evidence of a clinical difference between once-daily and twice-daily dosing, once-a-day regimens were likely to be preferable among both patients and physicians and, therefore, compliance was likely to be higher.
A study by Laliberté et al. suggests a clear patient preference for once-a-day-dosing. The investigators compared once-daily and twice-daily dosing regimens of chronic medications in nearly 6,000 patients with VTE. This showed that 45% of the once-a-day patients adhered to therapy compared with just 36% of those on twice a day.17
This conforms to the clinical experience of most of the meeting participants; although there is no gold standard for compliance, the perception of the panel was that taking medication once a day would be preferable to other dosage regimens.
Personal feedback from one of the panellists highlighted the almost universal patient preference for NOACs over warfarin for secondary prevention of transient ischaemic attack (TIA) or stroke at his hospital. Another contributor noted that it was the once-a-day dosing regimen that usually convinced patients to opt for NOAC treatment after being presented with the various options.
Practicalities of using anticoagulants
Several meeting participants pointed out that the once-a-day dosing also allowed for greater dosing flexibility, an important factor for those patients who might need to temporarily discontinue medication when undergoing surgery.
 Although formal guidelines should always be consulted prior to surgery, one suggestion from the panel was that the once-daily regimen has the potential to allow the patient to skip just one tablet before undergoing surgery the following day in some instances.
Although formal guidelines should always be consulted prior to surgery, one suggestion from the panel was that the once-daily regimen has the potential to allow the patient to skip just one tablet before undergoing surgery the following day in some instances.
Several meeting members expressed the belief that reversal agents for the NOACs would alleviate the fear of causing a bleed in a patient on anticoagulant therapy and would welcome these agents when they become available.
Missed dose
The meeting addressed the question of whether patients on a once-daily drug, rather than twice daily, might be at greater risk of a thrombotic event if they missed a dose. The general feeling was that this was of little concern.
It was pointed out that patients routinely came off their anticoagulant therapy during the peri-operative period and that the European Society of Cardiology (ESC) guidelines on the management of AF consider it “reasonable to undertake surgical or diagnostic procedures that carry a risk of bleeding in the presence of sub-therapeutic anticoagulation for up to 48h … given the low risk of thromboembolism in this period”.18
Several panellists noted the relative insignificance of a single missed dose in increasing stroke risk in patients; this relative insignificance was supported by another contributor, who drew on European guidance that patients undergo cardioversion back into sinus rhythm when they are in AF for up to 48 hours, even in the absence of any anticoagulation, indicating that one missed dose should not be a huge cause for concern. That said, the necessity of properly educating patients about the importance of adherence, and any potential consequences of non-adherence, should not be understated.
Pharmacological factors
Data from Mueck et al. was presented comparing the pharmacodynamics and pharmacokinetics of once-daily and twice-daily rivaroxaban in patients undergoing hip replacement surgery. These patients are at high risk of both VTE and post-operative bleeding. When comparing the same total daily doses, maximum plasma concentrations of rivaroxaban were higher and minimum plasma concentrations were lower with once-daily dosing, compared with twice daily; however, the 90% intervals overlapped. The area under the plasma concentration-time curve was 18–30% higher in the once-daily than in the twice-daily study. Prothrombin time in seconds (samples from 1,181 patients) correlated with rivaroxaban plasma concentrations in a linear fashion in both studies. In conclusion, the pharmacokinetics and pharmacodynamics of rivaroxaban were predictable when given either once or twice daily.19
 Participants discussed the pharmacological factors that distinguish a once-daily drug from a twice-daily drug. It was agreed that the important factors are the drugs:
Participants discussed the pharmacological factors that distinguish a once-daily drug from a twice-daily drug. It was agreed that the important factors are the drugs:
- Half-life – “important but not the whole story”
- Receptor binding – “long binding at the receptor can increase the duration of a drug way beyond its half-life”
- Dosage – “you can convert a twice-a-day drug into a once-a-day drug just by giving a higher dose”
- Bioavailability
- Therapeutic index – “if you’ve got a drug that causes side effects at a dose close to the therapeutic dose then you can’t give a higher dose”
- Metabolism and elimination – “both can fluctuate between individuals”.
Use of NOACs in the UK
NOACs currently account for 6% of the anticoagulation therapy prescribed in the UK.20 There are ‘pockets’ of higher uptake in the UK, such as Dudley in the West Midlands, where a large NHS stroke initiative has been underway for the past five years. This resulted in a large number of patients moving initially from aspirin therapy to warfarin. This, in turn, overwhelmed the region’s anticoagulation clinics and the decision was taken to place all poorly controlled patients on a NOAC.
Other pockets include Bradford, Bristol and parts of North Yorkshire, where GPs have started initiating rivaroxaban for newly diagnosed DVT.
The meeting considered some of the barriers to the greater use of NOACs. These included:
- Low awareness among the general public of the link between AF and stroke
- Resistance within Care Commissioning Groups (CCGs; where NOACs may be considered second-line therapy)
- ‘Policing’ by the prescribing advisers
- GPs’ preference for aspirin
- Quality and Outcomes Framework (QOF) incentive to use aspirin in AF – National Institute for Health and Care Excellence (NICE) guidance has in fact been updated in line with ESC guidance that aspirin should no longer be prescribed as monotherapy for patients considered at risk of stroke21
- A potential vested interest among some GPs and hospital anticoagulation clinics in warfarin monitoring
- The use of block contracts for anticoagulant services, making it difficult for CCGs to unbundle the costs of INR monitoring
- Pressure of time (“it takes a long time to switch a patient from warfarin to a NOAC”).
Cost benefit
While the greater cost of NOAC therapy compared with warfarin was perceived to be a barrier to increased use, the meeting acknowledged that these were likely to be offset by reduced monitoring costs. This has been confirmed in a study by Kourlaba of costs associated with moderate-to-high-risk AF patients on NOACs and VKAs. As an example, rivaroxaban therapy achieved a 0.22 increase in Quality Adjusted Life Year (QALY) and a saving of €239 lifetime costs compared with VKA therapy.22
One panellist added that if we study the places where NOACs are freely available, the rate of anticoagulation for AF is far greater than in other areas. The next step is to push the whole QIPP (Quality, Innovation, Productivity and Prevention) agenda for AF, recognising that anticoagulation is a priority not to be overlooked.
Conclusion
UK physicians are traditionally cautious in their adoption of radical new approaches to care. This certainly seems to be the case with the NOACs whose potential in the management of thromboembolic conditions remains largely untapped. Combined with a lack of awareness among the general population of the risks associated with AF and the longstanding underuse of thromboprophylaxis, it seems likely that large numbers of patients with potentially life-threatening conditions are being denied optimal care. This is not to discount the reasons for doctors’ circumspection. Concerns over bleeding risks, patient adherence, correct dosing and cost-effectiveness have long been a part of the management of SPAF, VTE and PE, and are likely to remain so. Nevertheless, data from clinical trials and ‘real-life’ registries, such as Dresden, suggest that the recently introduced anticoagulants have all of the pros of their predecessors with far fewer of the cons.5 As convincing data on cost-efficiency is recognised, it is likely that the commissioners of care will become increasingly receptive to the use of NOACs. After 50 years of warfarin, a new era may be about to begin.
References
1. Bauer KA. Pros and cons of new oral anticoagulants. ASH Educ Program Book 2013;2013:464–70. http://dx.doi.org/10.1182/asheducation-2013.1.464
2. No authors listed. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449–57. http://dx.doi.org/10.1001/archinte.1994.00420130036007
3. Shiyovich A, Khalameizer V, Katz A. New treatments for stroke and thromboembolism prevention in atrial fibrillation. Harefuah 2014;153:32–8, 64.
4. Asarcıklı LD, Şen T, İpek EG et al. Time in therapeutic range (TTR) value of patients who use warfarin and factors which influence TTR. J Am Coll Cardiol 2013;62(suppl 2):C127–C128. http://dx.doi.org/10.1016/j.jacc.2013.08.382
5. Beyer-Westendorf J, Förster K, Pannach S et al. Rates, management, and outcome of rivaroxaban bleeding in daily care: results from the Dresden NOAC registry. Blood 2014;124:955–62. http://dx.doi.org/10.1182/blood-2014-03-563577
6. Patel MR, Mahaffey KW, Garg J et al.; and the ROCKET AF Steering Committee for the ROCKET AF investigators. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N Engl J Med 2011;365:883–91. http://dx.doi.org/10.1056/NEJMoa1009638
7. EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510. http://dx.doi.org/10.1056/NEJMoa1007903
8. Capodanno D, Capranzano P, Giacchi G, Calvi V, Tamburino C. Novel oral anticoagulants versus warfarin in non-valvular atrial fibrillation: a meta-analysis of 50,578 patients. Int J Cardiol 2013;167:1237–41. http://dx.doi.org/10.1016/j.ijcard.2012.03.148
9. Lip GYH, Andreotti F, Fauchier L et al. Bleeding risk assessment and management in atrial fibrillation patients: a position document from the European Heart Rhythm Association, endorsed by the European Society of Cardiology Working Group on Thrombosis. Thromb Haemost 2011;106:997–1011. http://dx.doi.org/10.1160/TH11-10-0690
10. Halbritter K, Beyer-Westendorf J, Nowotny J, Pannach S, Kuhlisch E, Schellong SM. Hospitalization for vitamin-K-antagonist-related bleeding: treatment patterns and outcome. J Thromb Haemost 2013;11:651–9. http://dx.doi.org/10.1111/jth.12148
11. Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998;105:91–9. http://dx.doi.org/10.1016/S0002-9343(98)00198-3
12. Gitter MJ, Jaeger TM, Petterson TM, Gersh BJ, Silverstein MD. Bleeding and thromboembolism during anticoagulant therapy: A population-based study in Rochester, Minnesota. Mayo Clin Proc 1995;70:725–33. http://dx.doi.org/10.4065/70.8.725
13. Steffensen FH, Kristensen K, Ejlersen E, Dahlerup JF, Sorensen HT. Major haemorrhagic complications during oral anticoagulant therapy in a Danish population-based cohort. J Int Med 1997;242:497–503. http://dx.doi.org/10.1111/j.1365-2796.1997.tb00023.x
14. Willey VJ, Bullano MF, Hauch O et al. Management patterns and outcomes of patients with venous thromboembolism in the usual community practice setting. Clin Ther 2004;26:1149–59. http://dx.doi.org/10.1016/S0149-2918(04)90187-7
15. Gomes T, Mamdani MM, Holbrook AM, Paterson JM, Hellings C, Juurlink DN. Rates of hemorrhage during warfarin therapy for atrial fibrillation. CMAJ 2013;185:E121–E127. http://dx.doi.org/10.1503/cmaj.121218
16. Linkins L-A, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Int Med 2003;139:893–900. http://dx.doi.org/10.7326/0003-4819-139-11-200312020-00007
17. Laliberté F, Bookhart BK, Nelson WW et al. Impact of once-daily versus twice-daily dosing frequency on adherence to chronic medications among patients with venous thromboembolism. Patient 2013;6:213–24. http://dx.doi.org/10.1007/s40271-013-0020-5
18. European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery, Camm AJ et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–429. http://dx.doi.org/10.1093/eurheartj/ehq278
19. Mueck W, Borris LC, Dahl OE et al. Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost 2008;100:453–61. http://dx.doi.org/10.1160/TH07-12-0714
20. NHS England. Medicines Optimisation Dashboard. Available from: http://www.england.nhs.uk/ourwork/pe/mo-dash/ [accessed July 2014].
21. National Institute for Health and Care Excellence. Atrial fibrillation: the management of atrial fibrillation. NICE clinical guideline 180. London: NICE, June 2014. Available from: http://www.nice.org.uk/guidance/cg180
22. Kourlaba G, Maniadakis N, Andrikopoulos G, Vardas P. Economic evaluation of rivaroxaban in stroke prevention for patients with atrial fibrillation in Greece. Cost Eff Resour Alloc CE 2014;12(1):5. http://dx.doi.org/10.1186/1478-7547-12-5