Early identification of atrial fibrillation (AF), especially when asymptomatic, is increasingly important when there are interventions that can reduce the risk of stroke. One mobile ECG device that has the potential for doing just that is the AliveCor® device, which is non-invasive and easy to use. We aimed to assess its utility in primary care by establishing its sensitivity and specificity, and consider the predictive value for identifying AF in a general practice population.
We used the device on a population known to have AF in order to calculate the sensitivity, and on a population who did not have AF at the time of recording, to establish specificity. Using the known prevalence of AF in a UK population, we were able to calculate the predictive values for identification of AF. All AliveCor® traces we compared with a gold-standard 12-lead electrocardiogram (ECG).
The device has a high sensitivity and specificity in the hands of experienced clinicians. In particular, the sensitivity was consistently high, which would ensure a high true-positive rate of identification. Furthermore, the negative predictive value in populations with a prevalence of AF as in the UK is sufficiently high to be useful.
In conclusion, the AliveCor® device should be considered as an option for early identification of patients with unknown AF. It has a high negative-predictive value and is sufficiently sensitive to be useful in a general practice population, but does not rule out the need for a definitive ECG in suspected cases.
Introduction
People with atrial fibrillation (AF) are five times more likely to have a stroke.1 AF is an increasing problem as our population gets older.2 It is, therefore, important to be able to identify this condition as early as possible, when intervention with anticoagulation can prevent stroke, as is recommended by the National Institute for Health and Care Excellence (NICE), in most cases.3 Several studies have attempted to identify the most effective way of screening for, or case-finding, AF.4-7
The gold standard for diagnosis of AF is a 12-lead electrocardiogram (ECG). However, the 12-lead ECG is an impractical diagnostic tool for assessing those without symptoms, or without an apparently irregular pulse. Devices are increasingly being manufactured that could identify pre-symptomatic AF, such as WatchBP Home A device.8
One of the new devices that may be useful for case-finding asymptomatic people is the AliveCor® ‘App’ that can be used with a digital telephone (www.AliveCor.com). The AliveCor® device provides a single-lead ECG, which, if no ‘P-waves’ are identified or the rhythm is irregular, would trigger a 12-lead diagnostic ECG to be done. For it to be useful, it has to be able to demonstrate a sufficiently high sensitivity to identify cases, and a high negative-predictive value to be able to exclude cases in the primary care population. This means having a high specificity even in a relatively high prevalence population.
This study aims to establish the sensitivity and specificity of this device, and estimate the predictive values in an average general practice population.
The study was conducted independently of the manufacturer, who had no input into the purchase of the device, conception, design analysis or interpretation of the study.
Methods
To assess the sensitivity of the AliveCor® device, patients with known AF, diagnosed by 12-lead ECG, were recruited. To establish the specificity of the device, it was necessary to recruit patients without AF and confirm by 12-lead ECG.
The device was used to assess the rhythm of patients attending their regular AF clinic at the North West Heart Centre located at University Hospital South Manchester in Wythenshawe. Upon attending their outpatient appointment, the patients had a routine 12-lead ECG performed. At the same time, the patient was asked to use the AliveCor® device and a 30-second reading was taken using the application on the phone. A 12-lead ECG reading was recorded and printed at the same time. After the patient had concluded their ECG, they were allocated a number and their patient details removed from the 12-lead ECG for the purposes of blinding the readings for future interpretation. The AliveCor® reading was saved to a SD memory card once the reading had been converted into PDF format. Patient consent was established for conducting this study.
Alongside the known AF cohort of patients, were also many patients who were attending for reasons unrelated to AF. Those individuals, on whom the consultant wanted a 12-lead ECG performed in any case, were also asked to take a reading using the AliveCor® device. These patients underwent the same procedure as those with AF, and again were assigned a number, anonymised and their AliveCor® readings saved to memory card after conversion to PDF format.
Once the data had been collected, the 12-lead ECG recordings were then interpreted by a cardiac physiologist, used to reporting ECGs (KP) at the North West Heart Centre, and a general practitioner with a special interest in cardiology (IB). The recordings were designated as either being in AF or not. Once these had been interpreted, the AliveCor® readings were also assigned as either AF or not.
The designation of the 12-lead ECG was compared with the AliveCor® designation. The 12-lead ECG is regarded as the gold-standard diagnostic test for the purposes of this study. After the data regarding the readings had been analysed, it was then possible to calculate the sensitivity and specificity of the device. This was calculated by whether or not the AliveCor® reading corresponded to the 12-lead ECG designation. From this information it is possible to also calculate the positive- and negative-predictive values of the device depending on the prevalence of AF in any given population.
Results
In total, there were 99 patients who participated in the collection of ECG readings. Due to artefacts being present in some of the ECG recordings or the recordings were illegible, four patients were removed from the cohort. Therefore, the number of patients for analysis totalled 95. Of these 95 patients, 29 had AF using the 12-lead ECG as the method of interpretation, with 66 being in sinus rhythm.
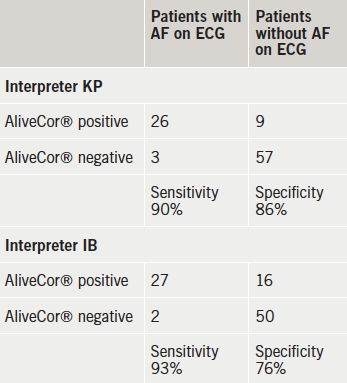
Of the 29 patients identified as being in AF via the 12-lead ECG recordings, using the AliveCor®, KP correctly identified 26 alongside a negative test for the remaining three individuals who were in AF according to the 12-lead ECG. With AliveCor®, IB identified nine with AF who did not have AF on the diagnostic 12-lead ECG, but identified 27 out of the 29 who did have AF on 12-lead ECG (table 1). Thus, in the case of KP the sensitivity of the AliveCor® device can be calculated as 90%, and the specificity as 86%. For IB the sensitivity was 93% and the specificity was 76%.
Discussion
The AliveCor® device is a simple and easy-to-use device. It is also lightweight and easily portable. The device can be used by professionals in primary care with minimal training. The diagnosis of AF is made by the absence of ‘P-waves’ and an irregularly spaced ‘QRS complex’. Even after potential identification with the AliveCor® device, there needs to be a diagnostic ECG.
The prevalence of AF on general practice registers in Central Manchester is 0.7%. This is lower than the national average of 1.5%, according to 2013 returns. However, much of the apparent under-ascertainment can be explained by the age distribution. The population of Central Manchester consists of 7.5% aged over 65 years compared with 17% over 65 years in England, according to 2011 census data. The prevalence of AF in over 65-year-olds in Central Manchester is likely to be similar to that throughout England.9
The high negative-predictive values suggest that this test is a good ‘rule-out’ test for AF in a population with a prevalence of AF, such as we have in England. A ‘positive’ AliveCor® test should still be combined with a 12-lead ECG to confirm the diagnosis of AF, but the test is more sensitive than assessing pulse regularity alone (77–84%).7
As the prevalence of AF increases with age, so too will the predictive values change. In one study,10 in the age range 75–79 years, the prevalence of AF was found to be 9%. So the positive-predictive value in this cohort is 39%, and the negative-predictive value is 99%. The prevalence of AF in the same study in those over 85 years was 18%. Therefore, the positive-predictive value in this cohort is about 59%, and the negative-predictive value in this cohort is about 98%. This prevalence is similar to a UK population, where the prevalence of AF in over 75-year-olds was 12%.6 In this population, the positive-predictive value will be about 47%, and the negative-predictive value will be 98%.
However, when case finding in a high-risk population, it is also important to have a low false-negative rate, in other words a high sensitivity. We do not want to miss cases. This may be sacrificed for a low false-positive rate, or specificity, since the final diagnosis will be made by a 12-lead ECG. For both analysers, an experienced electrophysiologist and a GP with a special interest in cardiology, the sensitivity is about 90%. In addition, in the population likely to be tested, the negative-predictive value is almost 100%.
Interpretation of the AliveCor® reading depends on the skill and experience of the clinician. So for less experienced clinicians, the sensitivity and specificity may be lower. This will lead to more false-positive and negative tests, and will result in more 12-lead ECGs being done than in experienced hands. This can be mitigated by a simple training programme to identify AF on the AliveCor® strip, or by commissioning a reporting service. The priority is to retain a high sensitivity, so that true positives are identified, which may be at the expense of a lower specificity.
The AliveCor® app can be used with a digital mobile telephone. It has a high sensitivity and negative-predictive value, which makes it potentially useful in case-finding AF, especially in the over 75-year-old population. It also has other uses within a cardiological assessment of patients. It should be considered as an option for use in early detection of AF.
Conflict of interest
None declared. The study was conceived, designed and conducted without any contribution from the manufacturer. The device was bought by IB.
Key messages
- AliveCor® is a simple and non-invasive way of identifying atrial fibrillation (AF)
- It has a high sensitivity in experienced hands and so will identify potential cases
- It has a high negative-predictive value in a general practice population and so is a good ‘rule-out’ test
- Suspected cases should have a gold-standard 12-lead electrocardiogram (ECG) as a diagnostic test
References
1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–8. http://dx.doi.org/10.1161/01.STR.22.8.983
2. Rothwell PM, Coull AJ, Giles MF et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 2004;363:1925–33. http://dx.doi.org/10.1016/S0140-6736(04)16405-2
3. National Institute for Health and Care Excellence. Atrial fibrillation: the management of atrial fibrillation. CG180. London: NICE, July 2014. Available from: http://www.nice.org.uk/guidance/cg180
4. Morgan S, Mant D. Randomised trial of two approaches to screening for atrial fibrillation in UK general practice. Br J Gen Pract 2002;52:373–80. Available from: http://bjgp.org/content/52/478/373
5. Sudlow M, Rodgers H, Kenny RA et al. Identification of patients with atrial fibrillation in general practice: a study of screening methods. BMJ 1998;317:327–8. http://dx.doi.org/10.1136/bmj.317.7154.327
6. Hobbs FD, Fitzmaurice DA, Mant J et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess 2005;9:iii–iv, ix–x, 1–74. http://dx.doi.org/10.3310/hta9400
7. Somerville S, Somerville J, Croft P, Lewis M. Atrial fibrillation: a comparison of methods to identify cases in general practice. Br J Gen Pract 2000;50:727–9. Available from: http://bjgp.org/content/50/458/727
8. National Institute for Health and Care Excellence. WatchBP Home A for opportunistically detecting atrial fibrillation during diagnosis and monitoring of hypertension. MTG13. London: NICE, January 2013. Available from: http://www.nice.org.uk/guidance/mtg13
9. Langenburg M, Hellemons BSP, van Ree JW et al. Atrial fibrillation in elderly patients: prevalence and comorbidity in general practice. BMJ 1996;313:1534. http://dx.doi.org/10.1136/bmj.313.7071.1534
10. Heeringa J, van der Kuip DA, Hofman A et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949–53. http://dx.doi.org/10.1093/eurheartj/ehi825

