High-risk atherosclerotic cardiovascular disease (ASCVD), including recent history of acute coronary syndrome, cerebrovascular atherosclerotic disease, peripheral arterial disease, and coronary artery disease with diabetes mellitus, requires lipid-level monitoring and treatment. Data for patients with high-risk ASCVD from 2008–2011 were obtained from the UK Clinical Practice Research Datalink. Across two years of follow-up, analyses examined lipid-altering drug use, statin adherence (medication possession ratio [MPR]), and persistence (continuous time on drug). Initial statin dose, upward dose titration, and use of high-intensity statins were also studied. Lipid levels were examined overall and by statin use. A total of 131,603 high-risk ASCVD patients were included. Within six months of diagnosis, 63.1% of patients received a statin prescription. Patients typically remained on the initial statin (MPR ≥80% for 71.3% of patients at two years) and dose; 16.4% of patients used high-intensity statins. During the first year of follow-up, 69.3% of patients were either at the low-density lipoprotein cholesterol goal of <2.5 mmol/L or using a high-intensity statin. Considerable room for improvement remains with respect to optimal management of patients with ASCVD.
Introduction
 Atherosclerotic cardiovascular disease (ASCVD), including peripheral arterial disease (PAD), coronary artery disease (CAD), acute coronary syndrome (ACS), and cerebrovascular disease (CeVAD), together account for approximately half of the morbidity and mortality in the adult population of Europe aged 50 years and older.1,2 The 2012 Coronary Heart Disease Statistics from the British Heart Foundation reported nearly 180,000 deaths in the UK from cardiovascular disease (CVD), 292 million prescriptions for CVD treatments, and over 87,000 percutaneous coronary interventions (PCIs) during a one-year span.3 In addition, diabetes mellitus (DM) has a prevalence of over 5% in the UK. Studies have found that roughly 27% to 58% of CAD patients also have DM, which is an independent risk factor for poor CVD outcomes.3-11
Atherosclerotic cardiovascular disease (ASCVD), including peripheral arterial disease (PAD), coronary artery disease (CAD), acute coronary syndrome (ACS), and cerebrovascular disease (CeVAD), together account for approximately half of the morbidity and mortality in the adult population of Europe aged 50 years and older.1,2 The 2012 Coronary Heart Disease Statistics from the British Heart Foundation reported nearly 180,000 deaths in the UK from cardiovascular disease (CVD), 292 million prescriptions for CVD treatments, and over 87,000 percutaneous coronary interventions (PCIs) during a one-year span.3 In addition, diabetes mellitus (DM) has a prevalence of over 5% in the UK. Studies have found that roughly 27% to 58% of CAD patients also have DM, which is an independent risk factor for poor CVD outcomes.3-11
Recommendations on the secondary prevention of CVD for patients with ASCVD have been published in guidelines from the National Institute for Health and Care Excellence (NICE).12,13 Statin therapy is recommended for adults with clinical evidence of CVD. The 2008 NICE guidelines suggested starting with simvastatin 40 mg for secondary prevention of cardiovascular events other than ACS and with a higher-intensity statin following ACS, increasing the dose to 80 mg simvastatin or a drug of similar efficacy and cost if lipid levels showed insufficient response.12 The 2014 guidelines, however, recommend atorvastatin 80 mg (or maximum tolerated dose) in place of simvastatin 40 mg for secondary prevention.13
The European Society of Cardiology (ESC) guidelines provide target low-density lipoprotein cholesterol (LDL-C) levels of <1.8 mmol/L for patients at very high risk of CVD (including post-ACS patients), and <2.5 mmol/L for patients at slightly lower but still high risk of cardiovascular events, including other ASCVD patients.14
The present study was designed to produce real-world, population-based evidence from the UK on the treatment patterns and lipid levels of a group of high-risk ASCVD patients, including patients with a recent history of ACS within the past year (hACS), CeVAD, PAD, and diabetes with confirmed CAD (DM/CAD), who are at elevated risk of having another cardiovascular event. For brevity, we, heretofore, use the simpler abbreviation of ASCVD to refer specifically to the high-risk ASCVD population of interest.
Methods
Data source
Data were obtained from the Clinical Practice Research Datalink (CPRD), a large database containing anonymised medical information on approximately 8% of the UK population from 630 general practices. At the time of this study, there were 11 million patients in the data repository, with 5.2 million current, active registrants and approximately 67 million patient-years of data. Information in the database includes diagnoses, procedures, referrals, lab tests with results, prescriptions, and demographics. The general practitioner (GP) data were linked to data from the Hospital Episode Statistics (HES) database, which was used to identify hospitalisation events in England, and to the Office for National Statistics mortality data.
Patient selection
Data were extracted for ASCVD patients (≥18 years of age) with a qualifying diagnosis from 1 April 2008 to 31 March 2011 (identification window), with a minimum of 12 months of continuous enrolment prior to a qualifying diagnosis in either CPRD or HES. ASCVD was defined as having a diagnosis of at least one of the following:
- hACS: ≥30 days through 365 days after discharge for ACS based on an ACS diagnosis in the HES data
- CeVAD: record in the GP or HES data indicating any CeVAD with or without ischaemic stroke
- PAD: GP or HES diagnosis, or an appropriate drug for treatment of PAD
- DM/CAD: GP or HES diagnosis of CAD recorded on or after the earliest date of diabetes, where diabetes was defined as having either a diagnosis of diabetes (either type 1 or type 2) or prescribed treatment for diabetes.
The index date was defined as the earliest diagnosis date of any of the above diseases within the identification window. Four patient groups were defined: hACS, CeVAD, PAD, and DM/CAD, according to the diagnosis that occurred on the index date.
Baseline characteristics
Patient characteristics included age, gender, body mass index (BMI), and smoking status. Baseline procedures for PCI and coronary artery bypass graft (CABG) were assessed from the 12 months prior to the index date. Comorbidities were coded using all available data from prior to the index date, as chronic conditions may not be re-recorded following initial diagnosis; these included diabetes, hypertension, hypercholesterolaemia, chronic obstructive pulmonary disease, renal impairment, hepatic impairment, congestive heart failure, metabolic syndrome, myopathy, atrial fibrillation, and the Charlson comorbidity index.15 Medication use assessed during the 12-month baseline included antiplatelets, anticoagulants, non-steroidal anti-inflammatory drugs, antidiabetic drugs, cardiovascular agents, and lipid-altering drugs.
Outcome variables
Use of lipid-lowering agents was noted in six-month blocks for the first two years of follow-up for each patient. The drugs examined included statins, ezetimibe, niacin, and fibrates, both alone and in combinations of a statin with another lipid-lowering drug. Combinations of other lipid-lowering agents with a statin required use of each of these at any time during the interval, not necessarily overlapping prescriptions.
For all patients treated with statins during the follow-up period, adherence, persistence, and dosing of the first statin received on or after the index date was tracked. The purpose of this analysis was to examine the use of statins beginning at the earliest date of qualification for the cohort; thus, patients were not required to be new users of a statin. Prevalent statin users were included, but their treatment patterns were assessed from only the index date forward. Patients prescribed more than one statin on the same day were excluded from these analyses.
Adherence was estimated with the medication possession ratio (MPR), calculated as the total days’ supply of prescribed medication during a given time period divided by the total number of days in that time period. Patients whose resulting MPR was over 100%, which can occur when a medication is refilled early, had their MPR truncated at 100%. The MPR was calculated for the first six, 12, and 24 months after the index date among patients enrolled for those durations. Persistence was estimated as the time from earliest statin use on or after the index date until the last date of exposure prior to a gap of more than 30 days; up to a 30-day prescription refill gap was considered continuous treatment.
To calculate the average daily dose, patients were required to have at least two prescriptions indicating at least daily use of the index statin. The prescribed doses of the index statin and any instance of high-intensity statin use were noted; atorvastatin 40–80 mg and rosuvastatin 20–40 mg were classified as high intensity, based on the high-intensity statin definition in the 2013 American College of Cardiology/American Heart Association guideline,16 which was the most recent guideline available at the time of the analysis. Upward dose titration was defined as any increase in dose over the initially prescribed dose.
Lipid levels were examined from the first and second years of follow-up separately and the first two years combined. The most favourable level (i.e. the lowest level, except for high-density lipoprotein cholesterol [HDL-C], where the highest level was most favourable) found during each interval for each patient was used. In addition to the numeric levels of each of these lab tests, levels were also categorised as follows:
1. Total cholesterol
- Ideal for CVD patients (<4.0 mmol/L or <155 mg/dL)3,14
- Recommended for CVD patients (4.0 to <4.5 mmol/L or 155–174 mg/dL)14
- Normal for patients without CVD but above recommended levels for CVD patients (4.5 to <5.0 mmol/L or 174–193 mg/dL)14
- Elevated (≥5 mmol/L or ≥193 mg/dL)14
- Unknown
2. LDL-C
- Recommended for non-ST-elevation myocardial infarction/unstable angina patients, ideal for PAD (<1.8 mmol/Lor 70 mg/dL) 14
- Recommended for other high-risk CVD patients (ST-elevation myocardial infarction [STEMI] patients with high risk) (1.8 to <2.0 mmol/L or 70–77 mg/dL)14
- Recommended for other CVD patients (STEMI) (2.0 to <2.5 mmol/L or>77–97 mg/dL)14
- Normal for patients without CVD but above recommended level for ASCVD (2.5–3.3 mmol/L or >97–128 mg/dL)17
- Slightly elevated (>3.3–4.1 mmol/L or >128–159 mg/dL)17
- High (>4.1–4.9 mmol/L or >159–189 mg/dL)17
- Very high (>4.9 mmol/L or >189 mg/dL)17
- Unknown
high-risk atherosclerotic cardiovascular disease (ASCVD) patients
3. HDL-C
- High risk for men and women (<1.0 mmol/L or 39 mg/dL)14
- High risk for women (1.0–1.2 mmol/L or 39–46 mg/dL)14
- Recommended (>1.2 mmol/L or >46 mg/dL)
- Unknown
4. Triglycerides
- Recommended (<1.7 mmol/L or <151 mg/dL)14
- Moderately high (1.7 to <10 mmol/L or 151 to <886 mg/dL)18
- Very high (10–20 mmol/L or 886–1,771 mg/dL)18
- Extremely high (>20 mmol/L or 1,771 mg/dL)18
- Unknown.
Statistical analysis
Persistence with the index statin was examined using the Kaplan-Meier median time to discontinuation; adherence was summarised as with the distribution of MPR values. Frequencies of initial dose were computed among patients with at least six months of follow-up, and of upward titration and prescription of high-intensity dose among patients followed for two years. Categories of lipid levels were examined among patients with known values, both overall and separately by statin use, high-intensity, non-high-intensity, and non-use. Analyses were descriptive only; no statistical comparisons were performed.
Results
Baseline characteristics
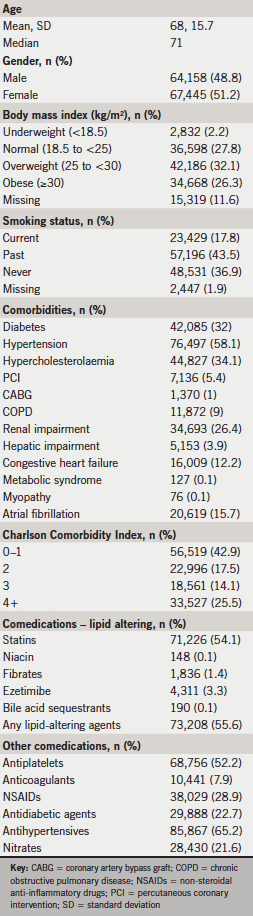
In total, 131,603 ASCVD patients met the inclusion criteria, including 16,629 patients with hACS, 34,044 with CeVAD, 54,492 with PAD, and 26,438 with DM/CAD. Median age was 71 years, and 48.8% of patients were male (table 1). Over half (58.4%) of the ASCVD cohort was overweight or obese, with 27.8% having normal BMI. Current smoking was reported for 17.8% of the cohort. Frequent comorbidities included hypertension (58.1%), hypercholesterolaemia (34.1%), diabetes (32.0%), and renal impairment (26.4%). Charlson Comorbidity Index scores were low (0–1) for 42.9% of the cohort, although about a quarter of patients (25.5%) had scores of 4 or higher. Antiplatelets were used by 52.2% of patients, and lipid-altering drugs, primarily statins, were used during baseline by 55.6% of patients.
Treatment patterns
During each six-month follow-up window, 63–65% of ASCVD patients were on any lipid-lowering medication, typically statin monotherapy (table 2). Ezetimibe was the most commonly used lipid-lowering drug other than a statin, but was used by <4% of patients.
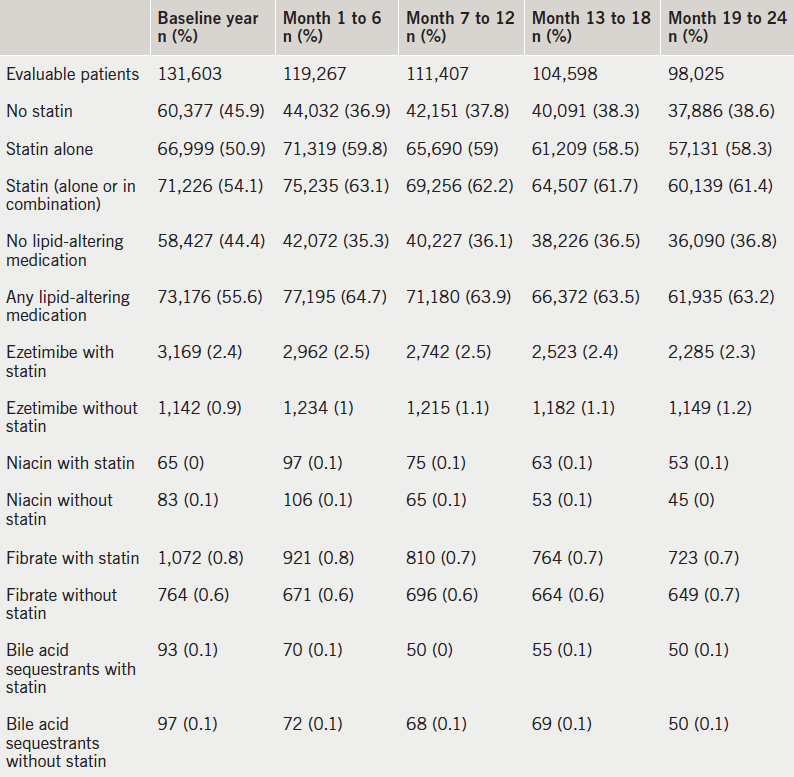
Simvastatin was by far the most commonly used statin (69.6%, n=44,227), followed by atorvastatin (23.3%, n=14,831) (table 3). Simvastatin was most often initiated at 40 mg per day (48.5% out of 63,549 eligible statin users); only 1,066 patients (1.7%) received the maximum allowable dose of 80 mg simvastatin. Atorvastatin 20 mg was prescribed to 5.3% of all patients; 8.2% received the 40 mg dose, and 5.4% the 80 mg dose, both of which were considered high-intensity levels. Across all prescriptions of the index statin across two years, atorvastatin was prescribed at high dose (40–80 mg) to 15.2% of all statin users, with rosuvastatin 20–40 mg prescribed to 1.2%. Overall, high-intensity statins (defined as atorvastatin 40–80 mg or rosuvastatin 20–40 mg) were prescribed to 16.4% of all statin users. Upward dose titrations were seen in 8.9% of patients overall, with little variation among drugs.

among statin users with ASCVD
Most patients (71.0%) remained on the index statin continuously (with no more than a 30-day gap) for the first six months of follow-up, leading to a Kaplan-Meier median days on treatment of the full time window (six months; table 4). High adherence, defined as an MPR ≥80%, was observed in 76.9% of patients at six months. Extending the time window to 12 months had little impact on the patterns of results. With a two-year time window, a Kaplan-Meier median time to discontinuation of the index statin was finally found, at 523 days. The percentage of patients with MPR ≥80% dropped only slightly from the six-month results, to 71.3%.

Lipid levels
Across the first two years combined, 80.2% of patients had total cholesterol levels recorded. Ideal levels (<4.0 mmol/L) were achieved by 48.2% of patients with known values, with another 18.9% at recommended levels (4.0 to <4.5 mmol/L) (table 5). Overall, 32.9% of patients with known lipid values had not achieved the recommended total cholesterol goal.

follow-up among ASCVD patients
LDL-C levels were available for 58.7% of patients, of whom 68.5% had LDL-C within recommended levels for general CVD patients (LDL-C <2.5 mmol/L) over the two-year period, and 34.6% had the stricter recommended goal of LDL-C <1.8 mmol/L (table 5). HDL-C was available for 71.2% of patients; 61.1% were within the recommended range of >1.2 mmol/L. Triglyceride levels were available for 64.6% of patients, with 74.3% having results within the recommended range (<1.7 mmol/L).
The ideal level of total cholesterol (<4.0 mmol/L) in the first year was found in 54.4% of patients on high-intensity statins, 50.4% on non-high-intensity statins, and 11.9% among statin non-users (table 6). The lowest category of LDL-C levels (<1.8 mmol/L) was seen in 41.3%, 35.5% and 5.3% of patients in the high-intensity, non-high-intensity and non-use statin groups in the first year, respectively. Differences in distribution of HDL-C and triglyceride levels across the three statin use groups were smaller than those of total cholesterol and LDL-C levels. Similar patterns were seen in the second year of follow-up.
During the first year of follow-up, 69.3% of patients (table 6) were either at the LDL-C goal of <2.5 mmol/L or using a high-intensity statin; the proportion was similar during the second year of follow-up (68.7%). Using the stricter recommended LDL-C level of <1.8 mmol/L, 40.2% of patients were either at the goal or were using a high-intensity statin (39.9% during the second year).
Discussion
In this large cohort of ASCVD patients from the UK, the majority of patients received statin treatment. Simvastatin was the most frequently prescribed statin and was generally used at the standard dose of 40 mg; when more intensive statin therapy was needed, it appears likely that patients were instead prescribed atorvastatin at high doses. Use of high-dose atorvastatin is consistent with the 2014 NICE guidelines,13 although the NICE guidelines in effect during the study period recommended either increasing the dose of simvastatin to 80 mg or changing to a higher-intensity statin.12 Use of simvastatin 80 mg may have already declined during the study period due to concerns regarding safety, especially myopathy and rhabdomyolysis.19 Overall, 16.4% of patients were prescribed high-intensity statins during two years of follow-up, and dose increases were seldom seen.

Patients usually remained on the same statin that was prescribed first on or after their date of diagnosis with the ASCVD condition. Adherence appeared excellent in these cohorts, with more than 70% of patients having an MPR of 80% or higher across two years of follow-up. Persistence with statins was also high, at a median of 523 days. An earlier study of statin treatment patterns in ACS patients using the same data source reported that 43% of patients remained on statin treatment at four-year follow-up.20 The current study found 44.7% persistence among ACS patients at two years, although the allowable gap duration in this study was only 30 days, compared to 145 days in the previous study.
Lipid levels, especially LDL-C, either were not consistently measured annually or were measured but not entered into the database. During the first year of follow-up, 69.3% of patients were either at the LDL-C goal of <2.5 mmol/L or using a high-intensity statin, while 40.2% of patients were either at the LDL-C goal of <1.8 mmol/L or using a high-intensity statin. These percentages remained stable across the second year, at 68.7% and 39.9%, respectively. Therefore, for over 30% of ASCVD patients, treatment of lipid level elevations is not meeting any guideline recommendations, placing patients at greater risk of suffering further cardiovascular events.
Data in the CPRD should be complete with respect to events in the GP’s clinic, but data on hospitalisations and specialist visits may be missing or incomplete. The HES data contain hospitalisation data only for England. Thus, the hACS patients, identified only through the HES data, represent patients from England only, in contrast with the other patient groups. Drug use is recorded at the prescription level; patients may not have filled the prescriptions and taken the drug as prescribed, and dose titrations may be incompletely recorded. Measures such as lipid levels and BMI were available only when these measurements were taken and entered into the database.
The study cohort included a combination of patients experiencing either their first or recurrent ASCVD events. Treatment patterns and lipid levels may differ following a recurrent event compared with a first event. In addition, the inclusion of prevalent users of statins in the adherence and persistence analyses may have biased results relative to use of a pure inception cohort; prevalent users were included to provide a more complete view of the treatment patterns following an ASCVD diagnosis.
Despite these limitations, the study has many important strengths. This study is the first, to our knowledge, to examine the treatment patterns and lipid levels in a broad cohort of ASCVD patients in the UK. The source population for the cohort is large and generally highly representative of the UK population. Each of the disease groups contained tens of thousands of patients, and data for these patients included relevant lab values, prescription drug information, comorbidities, and linked hospital and mortality data.
In conclusion, the present study found that statin use is highly prevalent among ASCVD patients in the UK. Adherence and persistence with statins appear excellent, based on the prescription data available in the database, yet optimal lipid levels were not consistently achieved. Greater use of high-intensity statin treatment may be needed in this high-risk population to reduce the risk of further CVD events.
Funding
This study and resultant manuscript were funded by Eli Lilly and Company, Indianapolis, IN, USA.
Conflict of interest
Antje Tockhorn, Yajun Zhu, and Zhenxiang Zhao are employees and shareholders of Eli Lilly and Company, Indianapolis, IN, USA. Beth Nordstrom and Robert Donaldson are current employees of Evidera, which provides consulting and other research services to pharmaceutical, device, government, and non-government organisations. William Engelman and Jenna Collins were Evidera employees who participated in research for this study. In their salaried positions, they work with a variety of companies and organisations and are precluded from receiving payment or honoraria directly from these organisations for services rendered. Evidera received funding from Eli Lilly and Company for their work on this project.
Key messages
- In a large cohort of UK patients with high-risk atherosclerotic cardiovascular disease (ASCVD), 63% received statin therapy within six months of diagnosis
- Dose titration of statins or switching among different statins were seldom seen, with 71% of patients having at least 80% statin adherence across two years
- More than 30% of patients had lipid levels above the recommended level and were not taking high-intensity statins
- Considerable room for improvement remains with respect to optimal management of patients with high-risk ASCVD
References
1. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 1997;349:1498–504. http://dx.doi.org/10.1016/S0140-6736(96)07492-2
2. Nichols M, Townsend N, Luengo-Fernandez R et al. European Cardiovascular Disease Statistics 2012. European Heart Network, Brussels, European Society of Cardiology, Sophia Antipolis; September 2012. Available from: http://www.escardio.org/about/Documents/EU-cardiovascular-disease-statistics-2012.pdf (accessed 28 February 2013).
3. British Heart Foundation, Townsend N, Wickramasinghe K et al. Coronary heart disease statistics, 2012 edition. London: British Heart Foundation, December 2012. Available from: https://www.bhf.org.uk/publications/view-publication.aspx?ps=1002097 (accessed 21 February 2014).
4. Krempf M, Parhofer KG, Steg PG et al. Cardiovascular event rates in diabetic and nondiabetic individuals with and without established atherothrombosis (from the REduction of Atherothrombosis for Continued Health [REACH] Registry). Am J Cardiol 2010;105:667–71. http://dx.doi.org/10.1016/j.amjcard.2009.10.048
5. Turpie AG. Burden of disease: medical and economic impact of acute coronary syndromes. Am J Manag Care 2006;12(16 suppl):S430–S434. Available from: http://www.ajmc.com/journals/supplement/2006/2006-12-vol12-n16Suppl/Dec06-2422pS430-S434/
6. Odden MC, Coxson PG, Moran A et al. The impact of the aging population on coronary heart disease in the United States. Am J Med 2011;124:827–33.e5. http://dx.doi.org/10.1016/j.amjmed.2011.04.010
7. Mahoney EM, Wang K, Cohen DJ et al. One-year costs in patients with a history of or at risk for atherothrombosis in the United States. Circ Cardiovasc Qual Outcomes 2008;1:38–45. http://dx.doi.org/10.1161/CIRCOUTCOMES.108.775247
8. Mahoney EM, Wang K, Keo HH et al. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes 2010;3:642–51. http://dx.doi.org/10.1161/CIRCOUTCOMES.109.930735
9. Fang J, Alderman MH. Impact of the increasing burden of diabetes on acute myocardial infarction in New York City: 1990–2000. Diabetes 2006;55:768–73. http://dx.doi.org/10.2337/diabetes.55.03.06.db05-1196
10. Fox CS, Sullivan L, D’Agostino RB Sr, Wilson PW. The significant effect of diabetes duration on coronary heart disease mortality: the Framingham Heart Study. Diabetes Care 2004;27:704–08. http://dx.doi.org/10.2337/diacare.27.3.704
11. Lee PG, Cigolle C, Blaum C. The co-occurrence of chronic diseases and geriatric syndromes: the health and retirement study. J Am Geriatr Soc 2009;57:511–16. http://dx.doi.org/10.1111/j.1532-5415.2008.02150.x
12. National Institute for Health and Care Excellence. NICE clinical guideline 67. Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. London: NICE, May 2008. Available from: http://www.nice.org.uk/nicemedia/pdf/CG067NICEGuideline.pdf (accessed 10 February 2014).
13. National Institute for Health and Care Excellence. NICE clinical guideline 181. Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. London: NICE, July 2014. Available from: http://www.nice.org.uk/guidance/cg181 (accessed 5 August 2014).
14. Perk J, De Backer G, Gohlke H et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2012;33:1635–701. http://dx.doi.org/10.1093/eurheartj/ehs092
15. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–19. http://dx.doi.org/10.1016/0895-4356(92)90133-8
16. Stone NJ, Robinson J, Lichtenstein AH et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934. http://dx.doi.org/10.1016/j.jacc.2013.11.002
17. Mayo Clinic Staff. Diseases and conditions. Cholesterol levels: what numbers should you aim for? Rochester, MN: Mayo Foundation for Medical Education and Research, September 2012. Available from: http://www.mayoclinic.org/diseases-conditions/high-blood-cholesterol/in-depth/cholesterol-levels/ART-20048245 (accessed 10 February 2014).
18. Merriman H. Controlling triglyceride levels can reduce risk of CVD and pancreatitis. Berkhamsted, UK: Guidelines in Practice, July 2010. http://www.eguidelines.co.uk/eguidelinesmain/gip/vol_13/jul_10/merriman_triglycerides_jul10.php#.UvknyD1dXTo (accessed 10 February 2014). *this link has expired. Please see Publisher’s note at end of reference section.
19. Study of the Effectiveness of Additional Reductions in Cholesterol Homocysteine Collaborative Group, Armitage J, Bowman L, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Erratum in Lancet 2011;377:126. Lancet 2010;376:1658–69. http://dx.doi.org/10.1016/S0140-6736(10)60310-8
20. Boggon R, Eaton S, Timmis A et al. Current prescribing of statins and persistence to statins following ACS in the UK: a MINAP/GPRD study. Br J Cardiol 2012;19:24. http://dx.doi.org/10.5837/bjc.2012.003
Publisher’s note
MGP, publishers of Guidelines in Practice, have advised us that the link in reference 18 has been replaced by: www.guidelinesinpractice.co.uk/gastrointestinal/controlling-triglyceride-levels-can-reduce-risk-of-cvd-and-pancreatitis/310775.article
