The past decade has seen the emergence of several new classes of drugs for the treatment of type 2 diabetes mellitus (T2DM). Despite the increasing use of these agents, metformin and sulfonylureas remain the most commonly prescribed glucose-lowering drugs in people with T2DM. This reflects the National Institute for Health and Care Excellence (NICE) guideline from 2015 and the Scottish Intercollegiate Guidelines Network (SIGN) guideline from 2010, which recommended metformin as first-line treatment and sulfonylureas as the ‘usual’ second-line treatment for patients with T2DM. SIGN has recently provided an updated guideline on the pharmacological management of glycaemic control in people with T2DM. For the first time in UK guidelines, this recommends that in individuals with diabetes and cardiovascular disease, sodium-glucose co-transporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists with proven cardiovascular benefit (currently empagliflozin, canagliflozin and liraglutide) should be considered. It is anticipated that implementation of these new guidelines will lead to increased prescribing of these drugs in people with diabetes and cardiac disease, with reductions in prescribing of dipeptidyl peptidase-4 (DPP-4) inhibitors and other drugs in the GLP-1 receptor agonist class, where cardiovascular benefits have not been clearly demonstrated.
Introduction
Around 10 years ago the Food and Drug Administration (FDA) and European Medicines Agency (EMA) issued guidance to drug manufacturers requiring that they produce evidence to demonstrate that any new glucose-lowering therapy does not result in an ‘unacceptable increase’ in cardiovascular risk.1,2 Evidence should be collected through large clinical trial programmes involving patients at higher risk of cardiovascular events, both to ensure sufficient end points are detected, and so that there is a true representation of the population with T2DM. Usually, this will include a dedicated cardiovascular outcome trial (CVOT) with a randomised design and a minimum of two years’ follow-up.
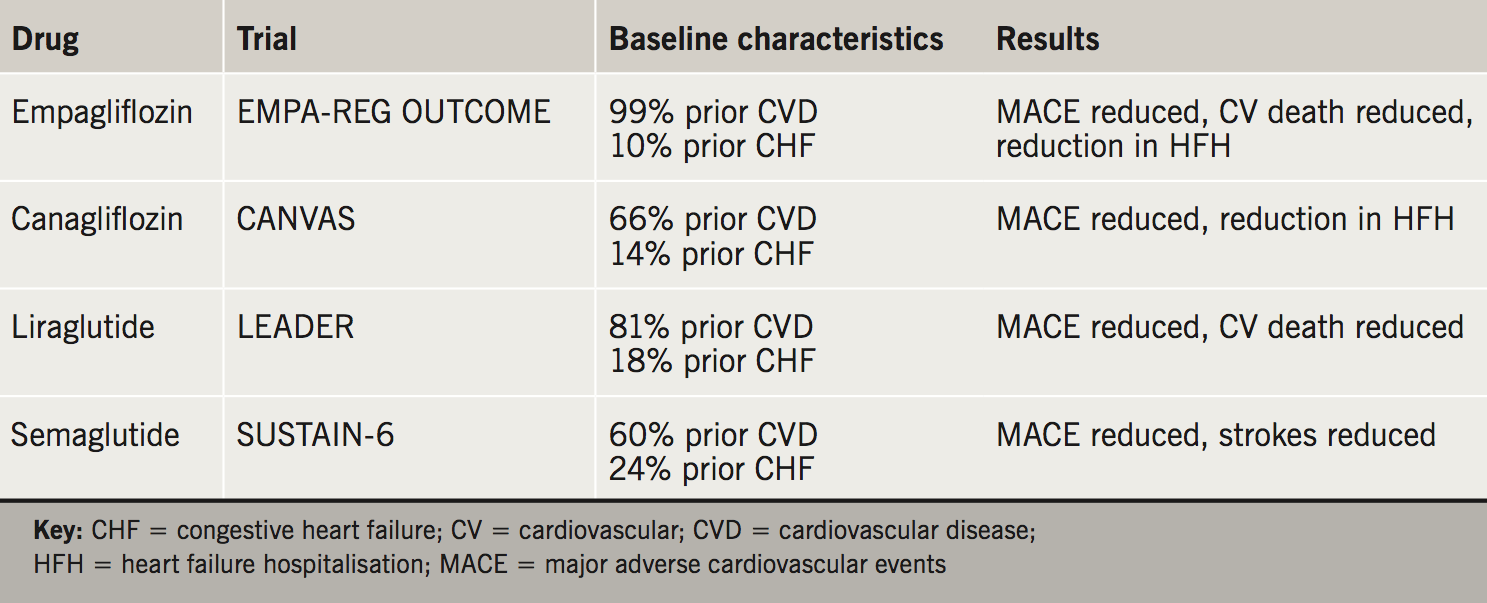
By the end of 2017 the results of 10 of these studies had been presented and published: three with dipeptidyl peptidase-4 (DPP-4) inhibitors,3-5 two with sodium-glucose co-transporter 2 (SGLT2) inhibitors,6,7 four with glucagon-like peptide-1 (GLP-1) receptor agonists,8-11 and one with insulin degludec,12 involving 94,799 people with T2DM. Four of these studies have shown significant reductions in major adverse cardiovascular events (MACE),6,7,9,10 and the remainder have demonstrated non-inferiority for MACE or MACE plus hospitalisation for unstable angina. The studies have included subjects with T2DM and recent acute coronary syndromes,4,8 patients with existing atherosclerotic disease,6 or a mixture of patients with existing cardiovascular disease and patients at high cardiovascular risk.3,5,7,9-12 The results of these studies can be used to inform treatment choices for the management of hyperglycaemia in these at-risk patients with diabetes depending on an accurate cardiovascular disease history (table 1).

Patients with existing atherosclerotic vascular disease
The FDA requirements for safety followed on from the rosiglitazone controversy, where rosiglitazone was suspected to have caused increases in nonfatal myocardial infarction (MI) and possibly cardiovascular death.13 The principle end point for the cardiovascular safety studies has either been MACE or MACE plus hospitalisation for unstable angina.14 The four studies that were positive and demonstrated superiority over placebo contained large numbers of patients with coronary artery disease, either in the form of stable coronary heart disease or previous MI (table 2). Of these therapies, the labels and summaries of product characteristics for empagliflozin and liraglutide have been updated to reflect the cardiovascular risk reductions demonstrated in EMPA-REG OUTCOME (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes Trial) and LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results), respectively. Semaglutide has recently been approved for licensing by the FDA and EMA, but has not yet been launched in Europe.
Currently, these drugs are prescribed for people with T2DM who have not reached their agreed, individualised glycaemic targets. In the future, prescribers may consider switching therapies in a patient who has already achieved their chosen target, but this is not common practice at present. For a patient with previous MI or stable coronary heart disease, who is not to target, the simplest intervention is the addition of a SGLT2 inhibitor, as this is an oral therapy. Empagliflozin is preferred over canagliflozin, which has been associated with increases in lower-limb amputations and fractures that have not been seen with empagliflozin.6,7 Liraglutide and semaglutide are injected subcutaneously, so the patient will require education from a trained healthcare professional, either in primary or secondary care, before starting these therapies. If the patient is on a DPP-4 inhibitor, this should be discontinued when starting a GLP-1 receptor agonist.
SGLT2 inhibitors and GLP-1 receptor agonists have different mechanisms of action, and a different pattern of cardiovascular benefits. SGLT2 inhibitors appear to exert their cardiovascular protective actions by haemodynamic or metabolic effects, while GLP-1 receptor agonists work via anti-atherogenic/anti-inflammatory mechanisms, raising the possibility that combined therapy with these two classes may produce additive cardiovascular benefits.15 There is a single published trial that compared the efficacy of dapagliflozin, exenatide once-weekly, and the combination of the two in patients who were not controlled on metformin monotherapy (DURATION-8).16 This demonstrated more pronounced improvement in glycosylated haemoglobin (HbA1c) and weight with combination therapy compared with either drug alone. Further efficacy comparisons between different SGLT2 inhibitors, GLP-1 receptor agonists and combinations are in progress, but no comparative cardiovascular outcomes trials are planned.
Pioglitazone reduced further cardiovascular events in patients with established coronary artery disease in the PROactive study (PROspective pioglitAzone Clinical Trial In macroVascular Events),17 but pioglitazone is seldom initiated now as it is not well tolerated by patients because of weight gain and fluid retention, it increases the risk of hip fractures, and the weight reductions that are seen with SGLT2 inhibitors and GLP-1 receptor agonists offer alternatives that are preferred by patients in routine clinical practice.
Patients with heart failure
Hospitalisation for heart failure is not a component of the FDA primary end point and is usually included as a secondary outcome. Hospitalisation for heart failure is an increasingly common clinical occurrence in people with T2DM, and some have argued that as an end point it should receive equal prominence to atherosclerotic outcomes.18 A significant proportion of patients in the four positive studies had heart failure at baseline, and hospitalisation for heart failure was a common outcome (table 2). It is important to note that heart failure at baseline was reported by the individual clinical investigator, and there was no attempt to corroborate this clinical diagnosis at baseline, e.g. with echocardiography or measurement of B-type natriuretic peptide (BNP). By contrast, heart failure hospitalisation (HFH) as an end point was adjudicated blindly by an adjudication committee according to predefined criteria.
An unexpected result of the CVOTs with DPP-4 inhibitors was an increase in HFH with saxagliptin,3 and a subgroup of patients treated with alogliptin.4 The mechanism of this adverse effect is unknown at present, and these drugs should be avoided in patients with heart failure. This was not seen in the study with sitagliptin, which seems safe to use in that situation.5 Similarly, pioglitazone is contraindicated in patients with heart failure, as renal retention of salt and water causes fluid retention and worsening symptoms. Previous concerns regarding the safety of metformin in chronic heart failure have been discounted,19 and metformin is recommended by the European Society of Cardiology (ESC) as the first-line therapy for patients with T2DM and concomitant heart failure.20
In EMPA-REG OUTCOME and CANVAS (Canagliflozin Cardiovascular Assessment Study) the use of empagliflozin and canagliflozin, respectively, reduced hospitalisations for heart failure, whereas liraglutide and semaglutide had no significant effect on this outcome. Empagliflozin and canagliflozin are a reasonable second-line choice for patients with heart failure who are not controlled on metformin. They should only be initiated if the estimated glomerular filtration rate (eGFR) is over 60 ml/min/1.73m2, so are contraindicated in patients with heart failure and eGFR less than 60 ml/min/1.73m2. SGLT2 inhibitors can be started in the outpatient setting when the patient is clinically stable, as this situation mimics the use of these drugs in the outcomes studies. Dedicated studies with dapagliflozin and empagliflozin in heart failure populations are currently recruiting, including patients with heart failure who do not have T2DM.
At present, there are no published data on prescribing these drugs for patients with acute heart failure. SGLT2 inhibitors increase urine volume, reduce blood pressure and can precipitate a small but significant decline in renal function. Until more information is available, they should probably be avoided in acute heart failure when the patient will have rapid changes in fluid status, and may require titration of diuretics and neurohumoral antagonists, which reduce blood pressure and can cause hypotension and acute kidney injury as a side effect.
As indicated, liraglutide and semaglutide had no effect on HFH in the large CVOTs. There have been two small, dedicated trials of liraglutide in patients with heart failure. The first compared liraglutide with placebo in 300 patients with established heart failure, reduced left ventricular ejection fraction (LVEF) and recent hospitalisation for acute heart failure:21 59% of patients had T2DM at baseline. Over 25 weeks no significant effect was seen in various measures of clinical stability, and liraglutide did not lead to greater post-hospitalisation clinical stability. It was concluded that the findings did not support the use of liraglutide in that clinical situation. The second trial compared liraglutide with placebo in 241 patients with chronic heart failure and reduced LVEF who were clinically stable and on optimal heart failure treatment.22 After 24 weeks there was no difference in left ventricular systolic function, which was the primary end point of the study. Serious adverse cardiac events were seen in 12 patients (10%) treated with liraglutide compared with three (3%) in the placebo group. The authors concluded that more data on the safety of liraglutide in different subgroups of heart failure patients are needed.
A detailed description of heart failure outcomes in LEADER has not yet been published. In the main publication, patients without baseline congestive heart failure (CHF) had a significant reduction in MACE, whereas for patients with baseline CHF no reduction in MACE was demonstrated.9 Until more information is available, it seems sensible to avoid the initiation of a GLP-1 receptor agonist during an admission with acute heart failure, but to consider initiation in a patient who is clinically stable. As liraglutide can be prescribed in patients with chronic kidney disease (CKD), this can include patients with chronic heart failure and eGFR less than 60 ml/min/1.73m2.
Patients with an acute coronary syndrome
National and international guidelines no longer recommend the use of intensive intravenous insulin infusion following an acute coronary syndrome or MI. For example, the ESC 2017 guidelines for the management of patients presenting with ST-segment elevation MI (STEMI) simply recommend that glucose-lowering therapy should be considered for patients with glucose levels greater than 10 mmol/L, and that episodes of hypoglycaemia, defined as glucose less than 3.9 mmol/L, should be avoided.23
No benefit in MACE was seen when alogliptin was commenced between 15 and 90 days following an acute coronary syndrome, and similarly no benefit in MACE plus hospitalisation for unstable angina was seen when lixisenatide was commenced up to 180 days following an acute coronary syndrome. There are no published data on prescribing SGLT2 inhibitors at the time of an acute coronary syndrome. SGLT2 inhibitors lower blood pressure, and GLP-1 receptor agonists lower blood pressure and increase heart rate. Following an acute coronary syndrome, patients will be started on maximum secondary prevention including beta blockers and angiotensin-converting enzyme (ACE) inhibitors, both of which lower blood pressure. If SGLT2 inhibitors or GLP-1 receptor agonists are commenced at the time of an acute coronary syndrome the patient should be clinically stable and blood pressure will require careful monitoring, so it is probably safer to do this following discharge during cardiac rehabilitation rather than at the time of the acute admission.
Discussion
The evidence base for the cardiovascular benefits and side effects of individual antidiabetes drugs has expanded rapidly over the last few years and will expand further as more FDA mandated CVOTS are completed and reported. Several national and international guidelines have been updated, or are in the process of being updated, to reflect the results. The joint American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) position statement on the management of T2DM was last updated in 2015, before the positive results were available, and is currently being revised.24 The ADA has recently updated its standards of medical care in diabetes to suggest assessment for the presence of atherosclerotic cardiovascular disease. For patients with cardiovascular disease in whom glycaemic control is over target with metformin monotherapy, the addition of a second-line agent with evidence of cardiovascular risk reduction is recommended.25
In Scotland, the Scottish Intercollegiate Guidelines Network (SIGN) guideline on the pharmacological management of glycaemia control in people with T2DM has, as two of its key recommendations, that “in individuals with type 2 diabetes and established cardiovascular disease, SGLT2 inhibitors with proven cardiovascular benefit (currently empagliflozin and canagliflozin) should be considered” and “in individuals with type 2 diabetes and established cardiovascular disease, GLP-1 receptor agonist therapies with proven cardiovascular benefit (currently liraglutide) should be considered”.26 Once this starts to be implemented, it is anticipated that there will be increased prescribing of empagliflozin, canagliflozin and liraglutide, with a reduction in the prescribing of DPP-4 inhibitors and GLP-1 receptor agonists that have not clearly demonstrated these cardiovascular benefits.
The National Institute for Health and Care Excellence (NICE) guideline on the management of type 2 diabetes in adults was published in December 2015, after EMPA-REG OUTCOME was published, but made no recommendation based on the results.27 In May 2017, text was added on the use of SGLT2 inhibitors for initial drug treatment, but no recommendation was added on the use of specific drugs in patients with cardiovascular disease. NICE recently communicated to stakeholders that “NICE has found that there is insufficient evidence to make any further definitive recommendations, as currently trials have only reported on some of the drugs in the SGLT-2 and GLP-1 classes, with trials of others still ongoing. As such, we will not be publishing this guidance for consultation and the existing recommendations will remain. As new trial evidence becomes available NICE will consider the need to update this guidance. Stakeholders will continue to be kept informed at each stage of the process”. This is now out of step with other national guidelines, and it is hoped that NICE will reconsider this decision so that drugs that are proven to reduce cardiovascular events can be prescribed more widely to appropriate patients with T2DM.
Key messages
- Metformin remains the preferred first-line glucose-lowering therapy for patients with type 2 diabetes mellitus (T2DM) and cardiovascular disease including stable coronary artery disease, acute coronary syndromes and heart failure
- The sodium-glucose co-transporter 2 (SGLT2) inhibitors empagliflozin and canagliflozin, and the glucagon-like peptide-1 (GLP-1) receptor agonists liraglutide and semaglutide, have demonstrated improved cardiovascular outcomes for patients with T2DM and atherosclerotic cardiovascular disease, and are appropriate second-line agents in this population
- Empagliflozin and canagliflozin are appropriate second-line agents for patients with T2DM and heart failure; initiation should be avoided in decompensated heart failure and typically reserved for the outpatient setting
- No glucose-lowering therapy has demonstrated cardiovascular benefit in the acute post-myocardial infarction setting; metformin remains the usual first-line agent with consideration of SGLT2 inhibitors or GLP-1 receptor agonists in clinically stable patients
Conflict of interest
MF has received payments for lectures and advisory boards from Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Janssen, MSD, Napp, Novo Nordisk, Sanofi and Takeda. EJ: none declared. GM has received payments for lectures and advisory boards from Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Janssen, MSD, Napp, Novo Nordisk and Sanofi.
Editors’ note
This article is the sixth and final in our ‘Drugs for diabetes’ series. Previous articles have covered dipeptidyl peptidase-4 (DPP-4) inhibitors (doi:10.5837/bjc.2017.001), SGLT2 inhibitors (doi:10.5837/bjc.2017.010), glitazones (thiazolidinediones) (doi:10.5837/bjc.2017.018), Glucagon-like peptide-1 (GLP-1) receptor agonists (doi:10.5837/bjc.2017.030) and Older antidiabetic drugs (doi: 10.5837/bjc.2018.007).
References
1. Food and Drug Administration. Diabetes mellitus – evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. Guidance for Industry. Silver Spring, MD: Division of Drug Information, Center for Drug Evaluation and Research Food and Drug Administration, 2008. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf [accessed 29 January 2018]
2. European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. London: EMA, 2012. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf [accessed 29 January 2018].
3. Scirica BM, Bhatt DL, Braunwald E et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26. https://doi.org/10.1056/NEJMoa1307684
4. White WB, Cannon CP, Heller SR et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35. https://doi.org/10.1056/NEJMoa1305889
5. Green JB, Bethel MA, Armstrong PW et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42. https://doi.org/10.1056/NEJMoa1501352
6. Zinman B, Wanner C, Lachin JM et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. https://doi.org/10.1056/NEJMoa1504720
7. Neal B, Perkovic V, Mahaffey KW et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. https://doi.org/10.1056/NEJMoa1611925
8. Pfeffer MA, Claggett B, Diaz R et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–57. https://doi.org/10.1056/NEJMoa1509225
9. Marso SP, Daniles GH, Brown-Frandsen K et al. Liraglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:311–22. https://doi.org/10.1056/NEJMoa1603827
10. Marso SP, Bain SC, Consoli A et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44. https://doi.org/10.1056/NEJMoa1607141
11. Holman RR, Bethel MA, Mentz RJ et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–39. https://doi.org/10.1056/NEJMoa1612917
12. Marso SP, McGuire DK, Zinman B et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med 2017;377:723–32. https://doi.org/10.1056/NEJMoa1615692
13. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–71. https://doi.org/10.1056/NEJMoa072761
14. Marx N, McGuire DK, Perkovic V et al. Composite primary endpoints in cardiovascular outcomes trials involving type 2 diabetes patients: should unstable angina be included in the primary endpoint? Diabetes Care 2017;40:1144–51. https://doi.org/10.2337/dc17-0068
15. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT 2 inhibitor. Diabetes Obes Metab 2017;19:1353–62. https://doi.org/10.1111/dom.12982
16. Frias JP, Guja C, Hardy E et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, mulitcentre, double-blind, phase 3 randomised controlled trial. Lancet Diabetes Endocrinol 2016;4:1004–16. https://doi.org/10.1016/S2213-8587(16)30267-4
17. Dormandy JA, Charbonnel B, Eckland DJ et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–89. https://doi.org/10.1016/S0140-6736(05)67528-9
18. McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure in diabetes: an outcome that can no longer be ignored. Lancet Diabetes Endocrinol 2014;2:843–51. https://doi.org/10.1016/S2213-8587(14)70031-2
19. MacDonald MR, Eurich DT, Majumdar SR et al. Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested case-control study from the U.K. General Practice Research Database. Diabetes Care 2010;33:1213–18. https://doi.org/10.2337/dc09-2227
20. Ponikowski P, Voors AA, Anker SD et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–200. https://doi.org/10.1093/eurheartj/ehw128
21. Margulies KB, Hernandez AF, Redfield MM et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2016;316:500–08. https://doi.org/10.1001/jama.2016.10260
22. Jorsal A, Kistorp C, Holmager P et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE): a multicentre double-blind, randomised, placebo-controlled trial. Eur J Heart Fail 2017;19:69–77. https://doi.org/10.1002/ejhf.657
23. Ibanez B, James S, Agewall S et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–77. https://doi.org/10.1093/eurheartj/ehx393
24. Inzucchi SE, Bergenstall RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–9. https://doi.org/10.2337/dc14-2441
25. American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes 2018. Diabetes Care 2018;41(suppl 1):S73–S85. https://doi.org/10.2337/dc18-S008
26. Scottish Intercollegiate Guidelines Network. SIGN 154. Pharmacological management of glycaemic control in people with type 2 diabetes. Edinburgh: SIGN, November 2017. Available from: http://www.sign.ac.uk/assets/sign154.pdf [accessed 29 January 2018].
27. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. London: NICE, 2015. Available from: https://www.nice.org.uk/guidance/ng28 [accessed 29 January 2018].

