Recent advances in immune therapy for cancer have significantly improved the clinical outcomes of patients with advanced cancers, where prognosis has historically been very poor. With these new treatments have come new toxicities and, as the use of immunotherapy increases, we will see an increasing incidence of immune-related adverse events, with patients presenting as an emergency. It is important that all cardiologists, and other physicians who see these patients, are aware of life-threatening immune-related toxicities, in addition to their recommended investigation and treatment.
We describe a patient with acute cardiotoxicity secondary to immune therapy to illustrate the complexity of these adverse cardiovascular events, providing recommendations for screening, diagnosis and management.

Introduction
Immune checkpoint inhibitors (ICIs) have revolutionised outcomes in a number of advanced cancers. The median life-expectancy for patients with metastatic melanoma was nine months prior to the introduction of ICIs in 2011.1 One-year survival is now over 70%, and over half of patients are still alive at three years,2 with multiple phase II and III trials demonstrating durable responses in a range of tumour types.3 ICIs are antibodies that block the cytotoxic T-cell regulators lymphocyte-associated protein-4 (CTLA-4; ipilimumab), programmed cell death protein-1 (PD-1; nivolumab, pembrolizumab and cemiplimab) or the PD-1 ligand (atezolizumab, avelumab and durvalumab). They are now used as standard of care in malignant melanoma, non-small cell lung cancer, renal cell cancer, Hodgkin disease, hepatocellular carcinoma, gastro-oesophageal cancer, squamous cell carcinoma, and urothelial cancer, with ongoing trials in other cancers (table 1).
Table 1. Immune checkpoint inhibitors, molecular targets and clinical indications
| Drug | Trade name | Target | Examples of indications (FDA approved) |
|---|---|---|---|
| Ipilimumab* | Yervoy | CTLA4 | Malignant melanoma, renal cell cancer |
| Pembrolizumab | Keytruda | PD-1 | Malignant melanoma, NSCLC, head and neck squamous cell cancer, classic Hodgkin lymphoma, urothelial cancer, gastro-oesophageal cancer, cervical cancer, hepatocellular cancer, Merkel cell cancer, tumour agnostic approval for MSI high solid tumours |
| Nivolumab* | Opvido | PD-1 | Malignant melanoma, NSCLC, small-cell lung cancer, renal cell cancer, Hodgkin lymphoma, neck squamous cell cancer, urothelial cancer, hepatocellular cancer |
| Atezolizumab | Tecentriq | PD-L1 | Urothelial cancer, NSCLC |
| Durvalumab | Imfinzi | PD-L1 | Urothelial cancer, NSCLC |
| Avelumab | Bavencio | PD-L1 | Merkel cell cancer, urothelial cancer |
| *FDA approved combination therapy for melanoma and renal cell carcinoma Key: CTLA4 = cytotoxic T-cell regulators lymphocyte-associated protein-4; FDA = Food and Drug Administration; MSI = microsatellite instability; NSCLC = non-small cell lung cancer; PD-1 = programmed cell death protein-1; PD-L1 = programmed cell death protein-1 ligand |
|||
Recently published trials have shown that combination immune therapy is more effective than single-agent therapy, but there is evidence that this is associated with a greater risk of immune-related toxicity.4,5 Through stimulation of the cytotoxic T-cell-mediated pathways, the intended therapeutic target of cancer immunotherapy, ICIs have also been shown to cause a spectrum of immune reactions including dermatological, endocrine, gastrointestinal, hepatic, renal and respiratory toxicity with immune infiltration.
The pivotal ICI trials did not report any cardiovascular adverse events, probably because they were not powered to detect their low incidence. However, subsequent case reports have described lymphocytic infiltration of the myocardium and specialised conduction tissue, together with clinical symptoms including myocarditis, pericardial disease, heart failure, arrhythmias and vasculitis.6,7 Given the expanding indications of ICI therapy and the multiple combination studies, which are showing promising results, these agents are becoming part of the standard of care for the majority of cancer patients. In addition, combination therapies could potentially lead to an increase in cardiotoxicity incidence and severity, although there is currently limited evidence in support of this.8,9
Clinical case
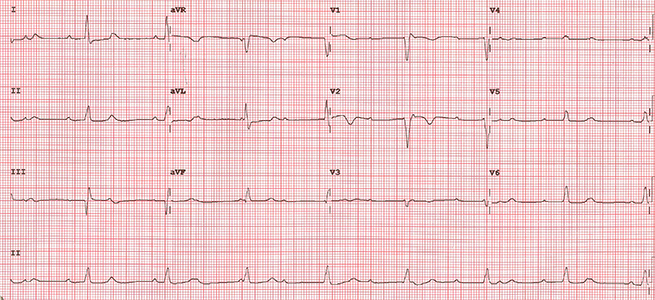
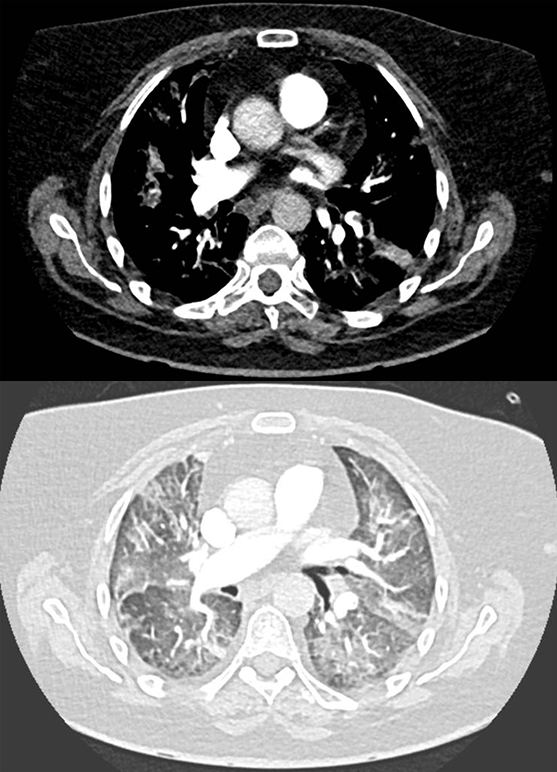
A fit 62-year-old woman presented with a right groin swelling. Histology was consistent with BRAF wild-type malignant melanoma and computed tomography (CT)-staging showed stage IV disease with widespread metastases. She was referred for systemic treatment and was given combination immunotherapy with the ICIs ipilimumab and nivolumab. She was seen in clinic after her first cycle where she was breathless, hypoxic (oxygen saturations 89% on room air) and bradycardic. Her electrocardiogram (ECG) showed complete heart block (figure 1) and a CT pulmonary angiogram showed bilateral segmental and sub-segmental pulmonary emboli with ground glass opacification consistent with pneumonitis (figure 2). Echocardiography showed normal left ventricular function and her high-sensitivity troponin-T was not elevated, N-terminal pro-brain naturietic peptide (pro-BNP) was not measured. A diagnosis of immunotherapy-related toxicity was made and she was started on intravenous steroids for the treatment of an ICI-related conduction abnormality and pneumonitis. A dual-chamber permanent pacemaker was implanted and she was anticoagulated.
Cardiotoxicity of immune checkpoint therapy
While clinical ICI-related cardiotoxicity is thought to be rare, its incidence may have been underestimated.10,11 As the number of patients receiving these treatments increases, it is important that cardiologists and other physicians recognise this new form of toxicity and start treatment promptly.7,12 The toxicity usually occurs early in the course of treatment with the median onset 30 days after the first dose.7,12 Not only is myocarditis more frequent with combined ipilimumab and nivolumab compared with nivolumab monotherapy (0.27% vs. 0.06%), dual therapy substantially increases the severity of cardiotoxicity with a mortality of 50%.7,10 The most common cardiovascular toxicity is myocarditis with an incidence of up to 1.3%, but arrhythmias including heart block and ventricular tachycardia, pericarditis and vasculitis also occur along with much more common non-cardiac toxicities including pneumonitis, colitis, hepatitis, thyroiditis, myositis, hypophysitis and dermatitis, which are reported as any grade treatment-related selected adverse event, occurring in 87.5% of patients receiving combination therapy with ipilimumab and nivolumab, 41.5% of whom have moderate or severe (grades 3–4) toxicity.13 With the exception of endocrinopathies, which require long-term hormone replacement therapy, the majority of non-cardiovascular complications resolve with corticosteroid therapy.12 ICI-induced myocarditis has also been associated with concurrent neuromuscular toxicity, presenting as either polyneuropathy, myasthenia gravis or myositis. Despite combined intravenous steroids and immunoglobulin therapy targeting this autoimmune toxicity, clinical outcomes detailed within case reports remain variable.14,15 Furthermore, physicians should be aware that cardiotoxicity from ICI-therapy can even present as a non-inflammatory diagnosis, such as conduction defects, left ventricular systolic dysfunction, Takotsubo cardiomyopathy and pericardial effusion, limiting the effectiveness of immunosuppressive therapy in these cases.16,17 Symptoms of immune-cardiotoxicity can be non-specific including chest pain, breathlessness, palpitations or blackouts. There are no randomised clinical trials of screening or treatment in immunotherapy-related cardiotoxicity. The general principles are to facilitate early detection and to start immunosuppressive therapy without delay (table 2).12,18
Table 2. Recommendations for evaluating cardiotoxicity in patients receiving immunotherapy12,18
| Prior to starting immunotherapy: Baseline ECG and troponin
|
| During immunotherapy Symptoms: chest pain, breathlessness, palpitations and blackouts or Signs: bradycardia, tachycardia, hypotension, oedema, pulmonary congestion
|
| Recommendations for management of suspected cardiotoxicity: Hold ICI therapy ECG, serum troponin, echocardiography Consider cardiac MRI if diagnosis is in doubt and the patient is clinically stable Endocardial biopsy may be considered if other investigations are inconclusive |
| If confirmed cardiotoxicity: Admit to hospital for urgent cardiology review Continuous ECG monitoring Methylprednisolone i.v. 2 mg/kg/day (Intravenous steroids should be commenced for suspected myocarditis if anticipated delay to diagnosis) Supportive treatment: heart failure with ACE inhibitor, beta blocker, diuretics, other therapy as indicated. If no improvement or severe symptoms consider methylprednisolone i.v. 1g/day and the addition of either mycophenolate, anti-thymocyte globulin or tacrolimus (infliximab is contraindicated at high doses in patients with moderate-severe heart failure) |
| Physicians should be aware of the clinical presentation of non-inflammatory cardiotoxicity, i.e. conduction defects, left ventricular systolic dysfunction, Takotsubo cardiomyopathy and pericardial effusion, particularly if patients fail to respond to high-dose steroid treatment |
|
Key: ACE = angiotensin-converting enzyme; ECG = electrocardiogram; ICI = immune checkpoint inhibitor; i.v. = intravenous; MRI = magnetic resonance imaging |
Diagnosing cardiotoxicity
If cardiotoxicity is suspected, ICI should be withheld and electrocardiogram (ECG), serum troponin and echocardiography performed. Although a normal troponin cannot exclude cardiotoxicity, observational reports suggest that up to 1% of patients receiving ICIs will have a raised troponin, with a final troponin T of ≥1.5 ng/ml associated with a four-fold increased risk of significant cardiovascular events.17 At present there is no evidence to suggest that surveillance of troponin in asymptomatic patients affects management, however, the availability of a baseline troponin can help to diagnose cardiotoxicity from ICI therapy. The role of brain natriuretic peptide (BNP) and N-terminal pro-BNP has also been evaluated, with elevated concentrations demonstrated in patients with confirmed ICI-mediated cardiotoxicity.16,17 Despite one cohort study revealing elevated BNP levels in 100% of patients (14 out of 14 patients), additional research has suggested that BNP may not rise in all patients with myocarditis, and may be influenced by concurrent cardiac therapy.16,17,19 Therefore, BNP should be measured in addition to troponin, to both support an inflammatory diagnosis and for cardiotoxicity surveillance. BNP may also serve a purpose in identifying non-inflammatory cardiotoxicity.
Echocardiography is recommended as the first-line investigation of ICI-associated myocarditis but normal left ventricular function and the absence of regional wall motion abnormalities do not exclude the diagnosis.12 Cardiac magnetic resonance imaging (CMR) with gadolinium enhancement is perhaps more useful for the diagnosis of ICI-induced cardiotoxicity, as it is more sensitive to the presence of myocardial inflammation and enables better tissue characterisation for myocarditis or fibrosis.11,20 Although positron emission tomography (PET) imaging with 18F-fluorodeoxyglucose (FDG) is rarely used in the diagnosis of myocarditis, emerging evidence recognises the value of this imaging modality for the detection of myocardial inflammation, with further research required to evaluate its integration with CMR.21,22
In cases where the diagnosis is in doubt, endomyocardial biopsy (EMB) should be considered to detect T-cell and macrophage infiltrates.7,11 However, given the potential for high false-negative rates due to the presence of patchy inflammatory infiltrates, delay in initiating treatment should be avoided in cases of suspected myocarditis, while the invasiveness of the procedure may also restrict its clinical value.20
Once a diagnosis is suspected, ICI should be withheld and ECG, serum troponin and echocardiography should be performed. If the diagnosis is confirmed, admission to hospital for cardiology review, ECG monitoring and intravenous corticosteroids is recommended, along with supportive therapy as indicated by the clinical situation.7,12,18
Our patient presented within 30 days of her first cycle of combination therapy with breathlessness and bradycardia. She had both cardiovascular and pulmonary toxicity with complete heart block attributed to myocarditis affecting her conduction system. It was not unexpected that her left ventricular function was normal at this stage, but it is less common for her serum troponin to have been normal, as reports suggest between 46%16 and 94%17 of patients with ICI-related myocarditis have significant troponin release. The differential diagnosis could, however, have been a non-inflammatory ICI-related conduction abnormality, given the lack of troponin rise and normal echocardiographic findings. Although not performed, CMR and/or EMB could have supported or refuted the diagnosis of myocarditis, while BNP may also have been of diagnostic value. She responded well to treatment and was discharged from hospital but unfortunately died of progressive disease three months later.
Key messages
- Immune checkpoint inhibitors have significantly improved response rates and patient outcomes across a wide range of cancers
- Cardiologists and oncologists need to be aware that immunotherapy has rare, but life-threatening cardiovascular (and multi-organ) toxicity
- Combination immunotherapy significantly increases tumour response rates but also the overall risk and severity of cardiotoxicity
- If cardiotoxicity is suspected, urgent electrocardiogram (ECG), serum troponin and echocardiogram can support the clinical diagnosis; cardiac magnetic resonance imaging (CMR) and endocardial biopsy may be considered if first-line investigations are inconclusive
- The mainstay of treatment for toxicity is prompt cessation of immunotherapy and initiation of intravenous steroids (e.g. 2 mg/kg/day methylprednisolone)
- As new indications and combinations of immunotherapy develop, the search for new biomarkers and optimal management strategies is essential
Conflicts of interest
SGF: none declared. RP has received fees for speaking at educational meetings and/or attending advisory boards from AstraZeneca, Bayer, BMS, Biosceptre, Clovis Oncology, CV6 Therapeutics, Cybrexa, Ellipses, Karus Therapeutics, Genmab, Octimet, Pierre Faber, Sanofi Aventis, and Tesaro; and a research grant from AstraZeneca. CP has received speaker’s fees from Amgen, Celgene, Ipsen, Novartis, Pfizer, and Roche.
Funding
None.
Consent
The patient details have been anonymised to respect confidentiality.
Editors’ note
See also the editorial by Georgiou and Yousaf in this issue.
References
1. Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14:463–82. https://doi.org/10.1038/nrclinonc.2017.43
2. Hodi FS, Chiarion-Sileni V, Gonzalez R et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:1480–92. https://doi.org/10.1016/S1470-2045(18)30700-9
3. Ellis PM, Vella ET, Ung YC. Immune checkpoint inhibitors for patients with advanced non-small-cell lung cancer: a systematic review. Clin Lung Cancer 2017;18:444.e1–459.e1. https://doi.org/10.1016/j.cllc.2017.02.001
4. Larkin J, Chiarion-Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. https://doi.org/10.1056/NEJMoa1504030
5. Postow MA, Chesney J, Pavlick AC et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17. https://doi.org/10.1056/NEJMoa1414428
6. Salem JE, Manouchehri A, Moey M et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579–89. https://doi.org/10.1016/S1470-2045(18)30608-9
7. Johnson DB, Balko JM, Compton ML et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–55. https://doi.org/10.1056/NEJMoa1609214
8. Gunnarsson O, Pfanzelter NR, Cohen RB, Keefe SM. Evaluating the safety and efficacy of axitinib in the treatment of advanced renal cell carcinoma. Cancer Manag Res 2015;7:65–73. https://doi.org/10.2147/CMAR.S74202
9. Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol 2017;29:84–91. https://doi.org/10.1093/annonc/mdx755
10. Varricchi G, Galdiero MR, Marone G et al. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2017;2:e000247. https://doi.org/10.1136/esmoopen-2017-000247
11. Wang DY, Okoye GD, Neilan TG, Johnson DB, Moslehi JJ. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep 2017;19:21. https://doi.org/10.1007/s11886-017-0835-0
12. Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. https://doi.org/10.1200/JCO.2017.77.6385
13. Sznol M, Ferrucci PF, Hogg D et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol 2017;35:3815–22. https://doi.org/10.1200/JCO.2016.72.1167
14. Rota E, Varese P, Agosti S et al. Concomitant myasthenia gravis, myositis, myocarditis and polyneuropathy, induced by immune-checkpoint inhibitors: a life-threatening continuum of neuromuscular and cardiac toxicity. eNeurologicalSci 2018;14:4–5. https://doi.org/10.1016/j.ensci.2018.11.023
15. Fazel M, Jedlowski PM. Severe myositis, myocarditis, and myasthenia gravis with elevated anti-striated muscle antibody following single dose of ipilimumab-nivolumab therapy in a patient with metastatic melanoma. Case Reports Immunol 2019;2019:3. https://doi.org/10.1155/2019/2539493
16. Escudier M, Cautela J, Malissen N et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 2017;136:2085–7. https://doi.org/10.1161/CIRCULATIONAHA.117.030571
17. Mahmood SS, Fradley MG, Cohen JV et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–64. https://doi.org/10.1016/j.jacc.2018.02.037
18. Hu JR, Florido R, Lipson EJ et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res 2019;115:854–68. https://doi.org/10.1093/cvr/cvz026
19. Ogawa T, Veinot JP, Kuroski de Bold ML, Georgalis T, de Bold AJ. Angiotensin II receptor antagonism reverts the selective cardiac BNP upregulation and secretion observed in myocarditis. Am J Physiol Heart Circ Physiol 2008;294:H2596–H2603. https://doi.org/10.1152/ajpheart.00215.2008
20. Upadhrasta S, Elias H, Patel K, Zheng L. Managing cardiotoxicity associated with immune checkpoint inhibitors. Chronic Dis Transl Med 2019;5:6–14. https://doi.org/10.1016/j.cdtm.2019.02.004
21. Haroon A, Zumla A, Bomanji J. Role of Fluorine 18 fluorodeoxyglucose positron emission tomography-computed tomography in focal and generalized infectious and inflammatory disorders. Clin Infect Dis 2012;54:1333–41. https://doi.org/10.1093/cid/cis193
22. Nensa F, Kloth J, Tezgah E et al. Feasibility of FDG-PET in myocarditis: comparison to CMR using integrated PET/MRI. J Nucl Cardiol 2018;25:785–94. https://doi.org/10.1007/s12350-016-0616-y
