Introduction
Peripheral artery disease (PAD) refers to all arterial disease outside of the coronary arteries and the aorta.1 It is estimated that over 200 million individuals are living with PAD globally.2,3 In the Western world, one in five adults over the age of 75 has PAD, including over 40 million Europeans.3-6 Though the prevalence of PAD is already at endemic levels worldwide, ageing populations and the increasing burden of chronic disease (i.e. hypertension, dyslipidaemia, diabetes mellitus, smoking) will contribute to further increases in the incidence and prevalence of PAD in the coming decades.2,3 As a consequence, PAD is the third leading cause of atherosclerotic morbidity and mortality behind coronary artery disease (CAD) and stroke.3,7
Unfortunately, the overall risk to patients with PAD is not solely due to the underlying peripheral arterial involvement. Patients with evidence of PAD are also at substantially increased risk of cardiovascular events in other vascular beds. For example, myocardial infarction (MI) and stroke may represent over 60% of deaths in patients with asymptomatic carotid atherosclerosis, thereby establishing the diagnosis of PAD as an important cardiovascular risk factor.8-10 Despite the abundance of PAD and its devastating consequences, PAD remains underdiagnosed and undertreated throughout Europe, highlighting a significant unmet clinical need.1,11 Below we review the clinical presentation of atherosclerotic PAD, the appropriate diagnostic investigations, and the available medical and surgical management options.
Pathophysiology and clinical presentation
PAD is frequently asymptomatic, though in a minority of cases acute arterial occlusion is the initial presentation. Abrupt occlusion of the carotid/vertebral, mesenteric, or extremity arteries can result in acute cerebrovascular events, acute mesenteric ischaemia, and acute limb ischaemia, respectively. These events are clinical emergencies with high morbidity and mortality requiring immediate diagnosis and treatment.1,7
In comparison, the majority of patients with indolent chronic atherosclerotic PAD present a far greater diagnostic challenge. The classical symptom of PAD – intermittent claudication – is an inherently unreliable indicator of the presence of disease.7 Studies have demonstrated that over 90% of individuals with PAD will not have classical symptoms. It is estimated that among patients with PAD, around 50% are likely to have atypical leg symptoms, and only 10–20% will present with intermittent claudication. Nevertheless, among asymptomatic patients with PAD up to 8% will have significant disease on non-invasive testing.3,4,12,13 Therefore, clinical screening of at-risk individuals supplemented by non-invasive testing may be necessary to adequately identify patients with PAD.
Diagnostic assessment
Clinical evaluation – history and examination
The initial evaluation of both symptomatic and asymptomatic PAD should attempt to ascertain cardiovascular risk through a detailed assessment of the patient’s symptoms and clinical history, in order to mitigate complications. Documenting the patient’s past medical history is crucial to identify important modifiable cardiovascular risk factors, such as known coronary or cerebrovascular disease, diabetes mellitus, aortic aneurysm, hypertension, dyslipidaemia, and chronic kidney disease.7 A family history of early onset PAD is associated with a three-fold increase in an individual’s risk, which is similar to the risk increase associated with smoking.14,15 Similarly, a detailed social history is crucial for evaluation of physical activity, dietary habits, and tobacco use, all of which have important management and prognostic implications in PAD.1
After establishing a patient’s risk factors for PAD, a thorough vascular examination is essential. This should include palpation of peripheral pulses in all four extremities, auscultation for carotid, renal and femoral bruits, examination of the lower extremities for evidence of chronic ischaemia (hair loss, non-healing wounds, ulcers, etc.) and bilateral blood pressure measurements.7,16 Though the sensitivity of physical exam findings for PAD is modest (58.2%), identification of a femoral bruit or any palpable pulse abnormality each have a likelihood ratio of more than four for the diagnosis of PAD.17,18 Both femoral and carotid bruits have been validated as independent risk factors for adverse cardiovascular events.1,19,20 In a meta-analysis of over 17,000 patients, the presence of a carotid bruit was associated with a two-fold increase in myocardial infarction (MI) and a two-and-a-half fold increase in cardiovascular death.19
Ankle-brachial pressure index and toe brachial pressure index
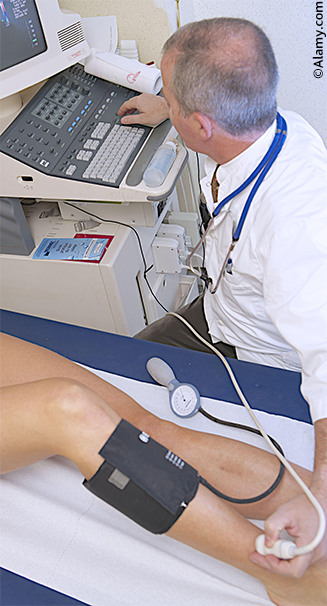
Following a comprehensive history and physical examination, patients suspected of having undiagnosed atherosclerotic PAD should undergo further non-invasive, confirmatory testing. The initial test of choice for diagnosing lower extremity PAD is the ankle-brachial index (ABI).1,7 The ABI is determined by measuring the systolic blood pressure in the patients brachial artery (both arms), while the patient is in the supine position, and dividing the highest measurement by the patient’s systolic blood pressure in their dorsalis pedis or posterior tibial artery (highest of the two).6,21 An ABI between 1.00 and 1.40 is considered in the normal range, and a value <0.90 is considered diagnostic of PAD.22 Calcified vessels that are unable to be appropriately compressed, which are present in 80% of patients with diabetes and 20% of patients without diabetes, can result in erroneously elevated ABI measurements.23 Further testing is therefore required in patients with an ABI of >1.40 and, with a normal ABI in those patients with diabetes, prior to PAD being excluded.6,7 In these instances obtaining a toe-brachial pressure index (TBPI) or a direct toe systolic pressure measurement with waveform analysis has been proven more reliable.24,25 Patients with an ABI between 0.90 and 1.00 also require further diagnostic testing, which may be in the form of post-exercise ABIs or imaging as discussed below.
Importantly, while the guidelines from the European Society of Cardiology (ESC) and the National Institute for Health and Care Excellence (NICE) both recommend ABI as the initial diagnostic investigation in individuals with suspected PAD, this should not be construed as a screening recommendation for all individuals. In a recent systematic review for the US Preventative Services Task Force, the authors found that diagnostic accuracy of ABI in an unselected population had a sensitivity of only 7–34%, compared with magnetic resonance angiography (MRA), and is therefore not adequately sensitive to screen for PAD.26 Its lower cost and powerful prognostic utility, however, justifies its place as the first tool for non-invasive evaluation of patients with suspected PAD.27,28
Imaging techniques
Multiple imaging modalities have established effectiveness in diagnosing PAD and assessing appropriateness for revascularisation. Currently accepted modalities, which include duplex ultrasound (DUS), computed tomography angiography (CTA), and MRA, are all able to identify, localise, and assess the severity of vascular lesions.1,6,7 Accordingly, the choice of modality must take into consideration both patient-related and non-patient factors. Patient-related factors include radiation exposure (CTA), contrast agent allergies (iodine-CTA), chronic kidney disease precluding contrast agents (iodine-CTA, gadolinium-MRA), and presence of implanted devices (e.g. permanent pacemakers, implantable cardioverter/defibrillators) that may be contraindicated in an MRI. Non-patient factors include availability of the test, local expertise, and cost.1,7 Based on these considerations, the ESC and the NICE guidelines recommend DUS as the first-line imaging technique in patients being considered for revascularisation, and MRA in those who require further imaging.1,6
Improving prognosis
As outlined above, patients with PAD are at increased risk of cardiovascular events in vascular territories outside of those with documented disease. The importance of identifying and treating concomitant risk factors cannot be overstated. Unfortunately, several previous investigations have demonstrated that PAD patients are less likely to receive aggressive medical management than patients with cerebrovascular or coronary artery disease.11,29 Once the diagnosis of PAD has been established, a comprehensive treatment strategy that focuses on risk factor modification and improving prognosis should be promptly instituted.
Risk factor modification
Risk factor reduction begins with non-pharmacological lifestyle modification strategies that must be tailored to each patient’s specific risk factors. Smoking is one of the most common risk factors among patients with PAD; an extensive body of literature has demonstrated the association between smoking and cardiovascular events, including an increased risk of amputation.30–32 However, the evidence has also demonstrated that smoking cessation leads to a rapid reduction in cardiovascular risk.30 Additional lifestyle modifications such as maintaining a healthy weight, regular physical exercise, and a healthy diet are also important.33
Pharmacologic treatments to reduce cardiovascular risk in patients with PAD are primarily targeted at three diseases: hypertension, diabetes mellitus, and dyslipidaemia. All patients with PAD should aim for a blood pressure goal of at least <140/90 mmHg in order to reduce cardiovascular events; angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB) should be considered as a first-line therapy, based on evidence supporting a reduction in cardiovascular events in patients with PAD.1,34–37
Nevertheless, the appropriate antihypertensive choice should also take into consideration any other comorbid conditions a patient might have (heart failure, CAD, or chronic kidney disease). In patients with diabetes, strict glycaemic control (HbA1c <7%) is strongly recommended through coordination of the healthcare team.1,7
The current ESC guidelines recommend that all patients with PAD should maintain low-density lipoprotein (LDL) cholesterol <1.8 mmol/L.1,32 Statins should be the primary pharmaceutical treatment used to achieve this LDL target based on extensive literature demonstrating an increase in maximal walking distance, and a reduction in both cardiovascular events and all-cause mortality.38–42 In patients unable to tolerate statin therapy, or unable to reach target LDL-cholesterol goals on maximally tolerated statin doses, clinicians should consider adding ezetimibe or a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor based on recent evidence demonstrating a reduction in cardiovascular events.43,44
Antithrombotic therapies
Antiplatelet agents
Antiplatelet therapy, with either aspirin (ASA 75–325 mg/day orally) or clopidogrel (75 mg/day orally) is recommended in all patients with symptomatic PAD, as well as those with asymptomatic carotid artery stenosis to reduce the risk of cardiovascular events.1 Evidence supporting the efficacy of ASA primarily comes from a significant reduction in major adverse cardiovascular events (MACE) in patients with PAD and intermittent claudication (ASA 6.4% vs. placebo 7.9%; p=0.004).45 These results were not reproducible in asymptomatic patients, thus antiplatelet therapy in patients with asymptomatic lower extremity PAD is not recommended.46,47 When compared with ASA, clopidogrel resulted in reduced cardiovascular mortality (HR 0.76 [95%CI 0.64 to 0.91]) and MACE (HR 0.78 [95%CI 0.65 to 0.93]) among symptomatic PAD patients.48
The EUCLID (Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease) trial randomised over 13,800 patients with symptomatic PAD to ticagrelor monotherapy (90 mg twice daily) or clopidogrel monotherapy (75 mg once daily). At a median follow-up of 30 months, ticagrelor failed to demonstrate a reduction in MACE (HR 1.02 [95%CI 0.92 to 1.13]; p=0.65), or acute limb ischaemia (HR 1.03 [95%CI 0.79 to 1.33]; p=0.49).49,50
Evidence supporting the utility of dual antiplatelet therapy (DAPT) for PAD is limited. The primary evidence for DAPT with clopidogrel comes from a post hoc analysis of the CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilisation, Management and Avoidance) trial, where symptomatic and asymptomatic patients with PAD had fewer MIs and strokes, but higher rates of bleeding.51 Similarly, a subanalysis of the DAPT trial, which randomised 11,648 patients 12-months post coronary stenting to an additional 18-month of DAPT therapy (ASA + clopidogrel or prasugrel) or ASA, found that the risk reduction with DAPT in PAD patients were similar to those observed in patients without PAD.52 Finally, in a substudy of the PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin) trial, investigators demonstrated that while PAD patients benefitted similarly from extended DAPT (ASA + ticagrelor) in terms of relative risk reduction, the absolute risk reduction was even greater.53 As these encouraging results are solely from post hoc analyses, current guidelines recommend against empiric DAPT, with the exception of patients who have undergone recent percutaneous revascularisation (carotid artery stenting, peripheral percutaneous stenting).1,7
Anticoagulants
Until recently, there has been no evidence to support anticoagulation in the management of PAD. Therefore previous guidelines did not recommend oral anticoagulation in the absence of an alternative indication.1,7 However, recent results from the COMPASS (Rivaroxaban for the Prevention of Cardiovascular Events in Coronary or Peripheral Artery Disease) trial – which evaluated the efficacy of rivaroxaban alone or in combination with ASA in secondary prevention of cardiovascular events – have demonstrated a reduction in MACE in patients receiving low-dose rivaroxaban (2.5 mg twice daily) combined with ASA (100 mg once daily).54 A substudy of patients with PAD confirmed the reduction in MACE in the group receiving low-dose rivaroxaban and ASA.55 Table 1 summarises ‘net clinical benefit’ end points from these analyses, comprising composites of adverse cardiovascular and bleeding outcomes. These data confirm that the combination of low-dose rivaroxaban + ASA provides superior net clinical benefit versus ASA alone.
Table 1. Summary of ‘net clinical benefit’ outcomes from the COMPASS trial
| Hazard ratio (95%CI) for stated treatment vs. aspirin alone | ||
|---|---|---|
| Main cohort (n=27,395) | PAD cohort (n=7,470)* | |
| Cardiovascular death, myocardial infarction, stroke, critical organ or fatal bleeding (prespecified end point) | ||
| Rivaroxaban 2.5 mg twice daily + aspirin | 0.80 (0.70 to 0.91), p<0.001 | 0.75 (0.60 to 0.94), p=0.011 |
| Rivaroxaban 5 mg twice daily alone# | 0.94 (0.84 to 1.07), p=0.36 | 0.92 (0.75 to 1.13), p=0.43 |
|
Compiled from data represented in Eikelboom JW et al.54 and Anand SS et al.55 Key: *patients in this cohort had evidence of peripheral artery disease (PAD) of the lower extremities (previous peripheral |
||
Managing symptoms
Exercise therapy
The mainstay of treatment among patients with chronic, non-limb threatening PAD is exercise therapy. The benefits of symptom improvement and quality of life have been well demonstrated in randomised trials, as well as a systematic review including over 1,800 patients.56 When compared to usual care, exercise therapy was found to increase the mean maximal walking time by 4.51 minutes (95%CI 3.11 to 5.92), the mean pain-free walking distance by 82.29 metres (95%CI 71.86 to 92.72), and the mean maximum walking distance by 108.99 metres (95%CI 38.20 to 179.78). Additional investigations demonstrated the benefits of supervised exercise therapy over unsupervised therapy, though both were superior to placebo.1,57 Based on these results, all patients with PAD should initially be managed with exercise therapy prior to considering additional pharmaceutical or revascularisation options.
Pharmaceutical treatments
Several agents have also been studied for their role in improving intermittent claudication symptoms. Cilostazol, naftidrofuryl, and pentoxifylline have the largest body of evidence. There have been two systematic reviews evaluating walking distance and quality of life measures with these medications as compared to placebo.42,58 Momsen et al. found that cilostazol, naftidrofuryl oxalate, and pentoxifylline all demonstrated significant improvements in maximal walking distance. These results, however, varied widely between studies, were relatively modest (60–90 metres), and were less robust than the improvements seen with statins (100 metres).42 In addition to improving walking distance, statins were found to significantly improve patients’ quality of life. When considered with the evidence discussed above demonstrating a reduction in cardiovascular events, statins become the obvious pharmaceutical option of choice in the initial management of symptomatic PAD.59
The role for cilostazol, naftidrofuryl, and pentoxifylline as second-line agents is somewhat controversial and societal guidelines provide varying recommendations. US guidelines recommend cilostazol as an effective treatment to improve symptoms and walking distance (Class I, Level A), and state that pentoxifylline is not effective in the treatment of claudication (Class III, Level B).7 Naftidrofuryl oxalate has not been approved in the USA. The NICE guidelines recommend naftidrofuryl oxalate only when supervised exercise has not led to satisfactory improvement in symptoms and the patient does not want to be referred for revascularisation.6 Finally, the ESC guidelines discuss the evidence for these medications but do not provide a recommendation for their use based on the variability in the available evidence, and the lack of evidence supporting their use in addition to statins.
Revascularisation
Only a very small percentage of symptomatic PAD patients will have critical limb ischaemia requiring revascularisation. Importantly, aside from symptomatic relief and quality of life improvement, revascularisation has not been shown to improve overall prognosis.6,7 Due to the subjectivity of claudication symptoms and the variable impact of these symptoms on an individual’s quality of life, decisions to pursue revascularisation must be individualised. Once individual patient factors such as comorbidities and surgical risk have been considered, the appropriateness to proceed with endovascular or surgical revascularisation should be undertaken in consultation with an expert. In general, percutaneous revascularisation options (balloon angioplasty, stenting, atherectomy) are preferred to surgery as the first-line strategy, though again this must be individualised to the patient and is dependent upon the specific anatomy (aorto-iliac vs. femoro-popliteal), the patient’s surgical risk, the likelihood of achieving a successful result, and previous treatment options (prior percutaneous procedures).6 If surgical bypass is the chosen strategy for infra-inguinal revascularisation, autologous vein grafts should be chosen over prosthetic grafts due to their superior patency rates.60,61
In the setting of critical limb ischaemia, a multidisciplinary vascular team should be involved in patient evaluation and management with the goal of achieving adequate revascularisation and minimal tissue loss. Whether surgical or percutaneous revascularisation offers an advantage over the other is still under investigation, though the available randomised studies suggest that these strategies offer comparable amputation-free survival.62,63 Amputation should be considered a last line of therapy in situations where all other revascularisation options have failed.6 Finally, acute limb ischaemia due to complete vascular occlusion is considered a medical emergency and necessitates rapid, time-sensitive evaluation and management, details of which are beyond the scope of this review.
Conclusion
PAD is clearly an important cause of cardiovascular morbidity and mortality throughout the world and its prevalence continues to rise. Regardless of symptoms, PAD places patients at substantially increased risk of adverse cardiovascular events. Consequently, treatment strategies aim to reduce patient symptoms while simultaneously improving prognosis. Until recently one major limitation to the currently available treatments was the lack of therapies providing a prognostic benefit in PAD. The results from the COMPASS trial suggest that low-dose rivaroxaban in combination with ASA may be able to fill this void and allow clinicians to take a significant step forward in the management of PAD in the 21st century.
Key messages
- Peripheral artery disease (PAD) is endemic throughout the world and its prevalence will continue to increase over the coming decades
- The majority of patients living with PAD are asymptomatic yet have a substantially greater cardiovascular risk than the general population
- Individuals at risk for PAD should undergo a thorough assessment of cardiovascular risk factors and a comprehensive vascular evaluation, followed by diagnostic testing in individuals requiring further assessment
- The main goals in the management of PAD are:
- improving patient prognosis through risk factor modification (i.e. smoking cessation and treatment of hypertension, diabetes, and dyslipidaemia) and antithrombotic therapies
- improvement of claudication symptoms through exercise therapy, pharmaceutical agents, and revascularisation
Conflicts of interest
None declared.
Jeffrey A Marbach
Interventional Cardiology Fellow
Aws S Almufleh
Cardiology Fellow (CAPITAL Research Group) and Advanced Heart Failure and Transplant Cardiology Fellow (Brigham and Women’s Hospital, Harvard Medical School)
Derek So
Consultant Interventional Cardiologist
Ann-Yeong Chong
Consultant Interventional Cardiologist
Email: (achong@ottawaheart.ca)
CAPITAL Research Group, University of Ottawa Heart Institute, 40 Ruskin Street, Ottawa, K1Y 4W7, Ontario, Canada
Articles in this supplement
Introduction
Atherosclerotic peripheral artery disease: the growing challenge to improve life and limb
Peripheral artery disease: current diagnosis and management
Combining rivaroxaban with aspirin in stable atherosclerotic vascular disease: clinical evidence from the COMPASS study
![]() Once you have read all these articles, you can take the ‘Learning with reflection’ CPD activity on this supplement.
Once you have read all these articles, you can take the ‘Learning with reflection’ CPD activity on this supplement.
References
1. Ricco J-B, Bartelink M-LEL, Bjorck M et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–821. https://doi.org/10.1093/eurheartj/ehx095
2. Hirsch AT, Duval S. The global pandemic of peripheral artery disease. Lancet 2013;382:1312–4. https://doi.org/10.1016/S0140-6736(13)61576-7
3. Fowkes FGR, Rudan D, Rudan I et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–40. https://doi.org/10.1016/S0140-6736(13)61249-0
4. Fowkes FG, Housley E, Cawood EH et al. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991;20:384–92.
5. Leng GC, Lee AJ, Fowkers FGR et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol 1996;25:1172–81.
6. Layden J, Michaels J, Bermingham S, Higgins B, on behalf of the Guideline Development Group. Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ 2012;345:e4947. https://doi.org/10.1136/bmj.e4947
7. Gerhard-Herman MD, Gornik HL, Barrett C et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary. J Am Coll Cardiol 2017;69:1465–508. https://doi.org/10.1016/j.jacc.2016.11.008
8. Belcaro G, Nicolaides AN, Ramaswami G et al. Carotid and femoral ultrasound morphology screening and cardiovascular events in low risk subjects: a 10-year follow-up study (the CAFES-CAVE study(1)). Atherosclerosis 2001;156:379–87.
9. Giannopoulos A, Kakkos S, Abbott A et al. Long-term Mortality in Patients with Asymptomatic Carotid Stenosis: Implications for Statin Therapy. Eur J Vasc Endovasc Surg 2015;50:573–82. https://doi.org/10.1016/j.ejvs.2015.06.115
10. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509–26. https://doi.org/10.1161/CIRCRESAHA.116.303849
11. Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation 2011;124:17–23. https://doi.org/10.1177/1358863X12437600
12. Hirsch AT, Criqui MH, Treat-Jacobson D et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001;286:1317–24. https://doi.org/10.1001/jama.286.11.1317
13. Leng G, Fowkes F. The epidemiology of peripheral arterial disease. Vasc Med Rev 1993;4:5–18.
14. Valentine RJ, Guerra R, Stephan P et al. Family history is a major determinant of subclinical peripheral arterial disease in young adults. J Vasc Surg 2004;39:351–6. https://doi.org/10.1067/mva.2002.125848
15. Wassel CL, Loomba R, Ix JH et al. Family history of peripheral artery disease is associated with prevalence and severity of peripheral artery disease: the San Diego population study. J Am Coll Cardiol 2011;58:1386–92. https://doi.org/10.1016/j.jacc.2011.06.023
16. Santilli JD, Santilli SM. Chronic critical limb ischemia: diagnosis, treatment and prognosis. AFP 1999;59:1899.
17. Simel D, Khan N, Anand S, Simel D, Panju A. Peripheral arterial disease. In: Simel D, Rennie D, editors. The rational clinical examination: evidence-based clinical diagnosis. McGraw-Hill; 2009.
18. Armstrong DWJ, Tobin C, Matangi MF. The accuracy of the physical examination for the detection of lower extremity peripheral arterial disease. Can J Cardiol 2010;26:e346-350. https://doi.org/10.1016/S0828-282X(10)70467-0
19. Pickett CA, Jackson JL, Hemann BA, Atwood JE. Carotid bruits as a prognostic indicator of cardiovascular death and myocardial infarction: a meta-analysis. Lancet 2008;371:1587–94. https://doi.org/10.1016/S0140-6736(08)60691-1
20. Cournot M, Taraszkiewicz D, Cambou J-P et al. Additional prognostic value of physical examination, exercise testing, and arterial ultrasonography for coronary risk assessment in primary prevention. Am Heart J 2009;158:845–51. https://doi.org/10.1016/j.ahj.2009.08.017
21. McDermott MM, Mazor KM, Reed G et al. Attitudes and behavior of peripheral arterial disease patients toward influencing their physician’s prescription of cholesterol-lowering medication. Vasc Med 2010;15:83–90. https://doi.org/10.1177/1358863X09353653
22. Alavi A, Sibbald RG, Nabavizadeh R et al. Audible handheld Doppler ultrasound determines reliable and inexpensive exclusion of significant peripheral arterial disease. Vascular 2015;23:622–9. https://doi.org/10.1177/1708538114568703
23. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabet Care 2003;26:3333–41. https://doi.org/10.2337/diacare.26.12.3333
24. Fagley RE, Haney MF, Beraud A-S et al. Critical care basic ultrasound learning goals for american anesthesiology critical care trainees: recommendations from an expert group. Anesthesia & Analgesia 2015;120:1041–53. https://doi.org/10.1213/ANE.0000000000000652
25. Migliacci R, Nasorri R, Ricciarini P, Gresele P. Ankle-brachial index measured by palpation for the diagnosis of peripheral arterial disease. Fam Pract 2008;25:228–32. https://doi.org/10.1093/fampra/cmn035
26. Guirguis-Blake JM, Evans CV, Redmond N, Lin JS. Screening for peripheral artery disease using the ankle-brachial index: an updated systematic review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality (US), 2018. Available at http://www.ncbi.nlm.nih.gov/books/NBK526319 (last accessed February 2018)
27. Ankle Brachial Index Collaboration. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008;300:197–208. https://doi.org/10.1001/jama.300.2.197
28. Criqui MH, McClelland RL, McDermott MM et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2010;56:1506–12. https://doi.org/10.1016/j.jacc.2010.04.060
29. Krishnamurthy V, Munir K, Rectenwald JE et al. Contemporary outcomes with percutaneous vascular interventions for peripheral critical limb ischemia in those with and without poly-vascular disease. Vasc Med 2014;19:491–9. https://doi.org/10.1177/1358863X14552013
30. Bullen C. Impact of tobacco smoking and smoking cessation on cardiovascular risk and disease. Expert Rev Cardiovasc Ther 2008;6:883–95. https://doi.org/10.1586/14779072.6.6.883
31. Lim SS, Vos T, Flaxman AD et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224–60. https://doi.org/10.1016/S0140-6736(12)61766-8
32. Piepoli MF, Hoes AW, Agewall S et al. [2016 European guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts. Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation]. G Ital Cardiol (Rome) 2017;18:547–612. https://doi.org/10.1714/2729.27821
33. Graham I, Atar D, Borch-Johnsen K et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur J Cardiovasc Prev Rehabil 2007;14(suppl 2):E1-40. https://doi.org/10.1097/01.hjr.0000277984.31558.c4
34. Staessen JA, Thijs L, Gasowski J, Cells H, Fagard RH. Treatment of isolated systolic hypertension in the elderly: further evidence from the systolic hypertension in Europe (Syst-Eur) trial. Am J Cardiol 1998;82:20R-22R. https://doi.org/10.1016/S0002-9149(98)00752-8
35. Jaques H, National Institute for Health and Care Excellence (NICE). NICE guideline on hypertension. Eur Heart J 2013;34:406–8. https://doi.org/10.1093/eurheartj/ehs428
36. Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000;342:145–53. https://doi.org/10.1056/NEJM200001203420301
37. ONTARGET Investigators, Yusuf S, Teo KK et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–59. https://doi.org/10.1056/NEJMoa0801317
38. Aung PP, Maxwell HG, Jepson RG, Price JF, Leng GC. Lipid-lowering for peripheral arterial disease of the lower limb. Cochrane Database Syst Rev 2007;CD000123. https://doi.org/10.1002/14651858.CD000123.pub2
39. Antoniou GA, Fisher RK, Georgiadis GS, Antoniou SA, Torella F. Statin therapy in lower limb peripheral arterial disease: Systematic review and meta-analysis. Vascul Pharmacol 2014;63:79–87. https://doi.org/10.1016/j.vph.2014.09.001
40. Kumbhani DJ, Steg PG, Cannon CP et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J 2014;35:2864–72. https://doi.org/10.1093/eurheartj/ehu080
41. Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg 2007;45:645–54; discussion 653–4. https://doi.org/10.1016/j.jvs.2006.12.054
42. Momsen AH, Jensen MB, Norager CB, Madsen MR, Vestersgaard-Andersen T, Lindholt JS. Drug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomised controlled studies. Eur J Vasc Endovasc Surg 2009;38:463–74. https://doi.org/10.1016/j.ejvs.2009.06.002
43. Murphy SA, Cannon CP, Blazing MA et al. Reduction in total cardiovascular events with ezetimibe/simvastatin post-acute coronary syndrome: The IMPROVE-IT Trial. J Am Coll Cardiol 2016;67:353–61. https://doi.org/10.1016/j.jacc.2015.10.077
44. Sabatine MS, Giugliano RP, Keech AC et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22. https://doi.org/10.1056/NEJMoa1615664
45. Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86. https://doi.org/10.1136/bmj.324.7329.71
46. Fowkes FG, Price JF, Stewart MCW et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA 2010;303:841–8. https://doi.org/10.1001/jama.2010.221
47. Belch J, MacCuish A, Campbell I et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008;337:a1840. https://doi.org/10.1136/bmj.a1840
48. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996;348:1329–39. https://doi.org/10.1016/S0140-6736(96)09457-3
49. Hiatt WR, Fowkes FGR, Heizer G et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med 2017;376:32–40. https://doi.org/10.1056/NEJMoa1611688
50. Chong A-Y, So DY. Ticagrelor for the treatment of peripheral arterial disease. Expert Opin Investig Drugs 2014;23:1737–43. https://doi.org/10.1517/13543784.2014.974803
51. Cacoub PP, Bhatt DL, Steg PG, Topol EJ, Creager MA, CHARISMA Investigators. Patients with peripheral arterial disease in the CHARISMA trial. Eur Heart J 2009;30:192–201. https://doi.org/10.1093/eurheartj/ehn534
52. Secemsky EA, Yeh RW, Kereiakes DJ et al. Extended duration dual antiplatelet therapy after coronary stenting among patients with peripheral arterial disease. JACC: Cardiovasc Interv 2017;10:942–54. https://doi.org/10.1016/j.jcin.2017.02.013
53. Bonaca MP, Bhatt DL, Storey RF et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol 2016;67:2719–28. https://doi.org/10.1016/j.jacc.2016.03.524
54. Eikelboom JW, Connolly SJ, Bosch J et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–30. https://doi.org/10.1056/NEJMoa1709118
55. Anand SS, Bosch J, Eikelboom JW et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018;391:219–29. https://doi.org/10.1016/S0140-6736(17)32409-1
56. Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev 2014;CD000990. https://doi.org/10.1002/14651858.CD000990.pub4
57. Gommans LNM, Fokkenrood HJP, van Dalen HCW et al. Safety of supervised exercise therapy in patients with intermittent claudication. J Vasc Surg 2015;61:512-518.e2. https://doi.org/10.1016/j.jvs.2014.08.070
58. Stevens JW, Simpson E, Harnan S et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br J Surg 2012;99:1630–8. https://doi.org/10.1002/bjs.8895
59. Gargiulo G, Giugliano G, Brevetti L et al. Use of statins in lower extremity artery disease: a review. BMC Surg 2012;12(suppl 1):S15. https://doi.org/10.1186/1471-2482-12-S1-S15
60. Pereira CE, Albers M, Romiti M, Brochado-Neto FC, Pereira CAB. Meta-analysis of femoropopliteal bypass grafts for lower extremity arterial insufficiency. J Vasc Surg 2006;44:510–7. https://doi.org/10.1016/j.jvs.2006.04.054
61. Twine CP, McLain AD. Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst Rev 2010;CD001487. https://doi.org/10.1002/14651858.CD001487.pub2
62. Adam DJ, Beard JD, Cleveland T et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005;366:1925–34. https://doi.org/10.1016/S0140-6736(05)67704-5
63. Bradbury AW, Adam DJ, Bell J et al. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technol Assess 2010;14:1–210, iii–iv. https://doi.org/10.3310/hta14140
Notes on dosing recommendations from Xarelto® ▼ (rivaroxaban) SmPC (Summary of Product Characteristics)
Xarelto 2.5 mg twice daily, coadministered with a daily dose of 75–100 mg aspirin, is indicated for the prevention of atherothrombotic events in adult patients with coronary artery disease (CAD) or symptomatic peripheral artery disease (PAD) at high risk of ischaemic events.
The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial discussed in this supplement compared both Xarelto 2.5 mg twice-daily plus aspirin and also Xarelto 5 mg twice-daily without aspirin, versus aspirin alone. Results for both comparisons are provided reflecting the original study publication.
Please note, however, that Xarelto 5 mg twice-daily is not a licensed dosage regimen for the above, nor for any other therapeutic indication.

