From the time that the first cases were reported from Wuhan, China on the 31st December 2019,1 our knowledge of the clinical and virological associations of the novel coronavirus (COVID-19) has been evolving at a rapid pace. On 18th May 2020, COVID-19 had caused over 4.82 million cases worldwide and resulted in 316,959 deaths.2 Whilst the primary focus of management for patients with COVID-19 remains close monitoring of respiratory function, there have been high levels of cardiac dysfunction in emerging cross-sectional and observational analyses, suggesting the need for heightened awareness in patients who may require cardiac input as part of a multidisciplinary approach. We review the current data on the association of COVID-19 and the heart.
The virology of COVID-19
The COVID-19 virus shares the majority of its genome with a previously identified bat coronavirus species RaTG13.3,4 In a fashion similar to the SARS coronavirus from 2002/2003, COVID-19 enters the cell when its ‘spike protein’ interacts with the ACE2 protein in host cells, and allows passage into the cell (see figure 1). Though COVID-19 is, on the whole, most identifiable with the bat coronavirus RaTG13, the spike protein on its own is more akin to that seen in coronavirus species seen in the Malayan pangolin.4 The spike protein in the COVID-19 virus was found to have similar (albeit less) binding affinity for the ACE2 protein to the previous SARS CoV,5 and thus knowledge can be gained from studies involving the previous SARS CoV.
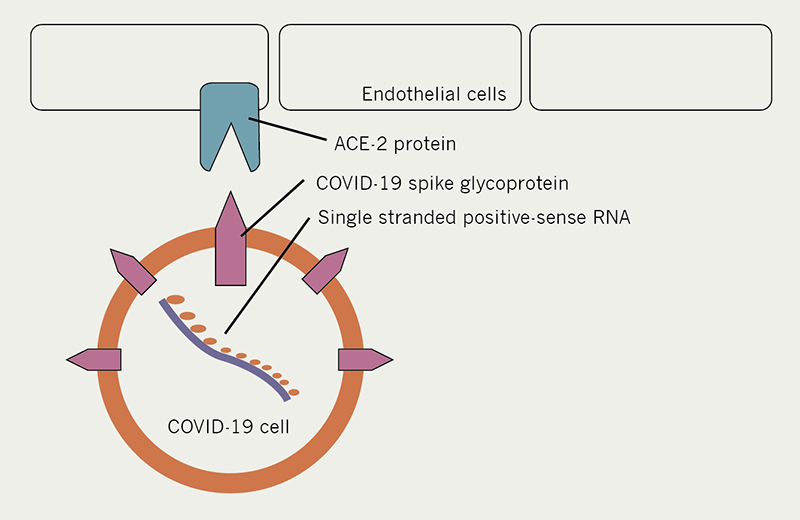
COVID-19 and the heart
In initial reports from China involving a cohort of 187 patients, COVID-19 was associated with a 16.7% incidence of arrhythmia and a 7.2% incidence of cardiac injury, rising to 44.4% and 61.1%, respectively, in patients who were admitted to intensive care.6 As previously mentioned, the ACE2 receptor has been established as the entry point of the COVID-19 virus to the cell,7–9 and this protein has been shown to be expressed by endothelial cells in the heart as well as the lungs.10,11
The invasion of coronaviruses into myocardial tissue has been associated with worse outcomes. In the case of SARS CoV, severe infection (requiring intubation) was associated with a lower left ventricular ejection fraction as compared with patients who did not require intubation. In an autopsy series from the 2003 SARS outbreak in Toronto, Canada, 35% of patients who died from SARS exhibited the presence of SARS-CoV RNA in myocardial tissue.12 Additionally, the presence of SARS Co-V in myocardial tissue was associated with an earlier death in these patients (3.9 days vs. 43.2 days, p=<0.05). In the patients where myocardial SARS CoV infiltration had taken place, ACE-2 protein expression was observed to be down-regulated.12
It is not known, however, whether the increased mortality associated with myocardial infiltration of the virus was due to the downstream reduction in angiotensin and thus its cardioprotective effects,10 or due to the TNF-alpha mediated profibrotic effects of the virus.12,13 This degree of analysis is not yet available with COVID-19, but due to the similarities in cellular mechanics it serves to provide a useful insight into likely mechanisms.
Cardiovascular risk factors
Since the first large report on patient factors with COVID-19 infection was published in March 2020 (n=191), it was clear that cardiovascular risk factors significantly predispose patients to worse outcomes with COVID-19 infection.14 The odds ratio of dying as an inpatient was increased for patients who were:
- older (odds ration [OR]1.10 per year increase, 95% confidence interval [CI] 1.03–1.17)
- had hypertension (OR 3.05, 95%CI 1.57–5.92)
- diabetes (OR 2.85 95%CI 1.35–6.05)
- or coronary artery disease (OR 21.4, 95%CI 4.64–98.76).
In this cohort, acute cardiac injury was reported to have occurred in 17% of patients (in 59% of non-survivors and 1% of survivors [p=<0.0001]) and heart failure occurred in 23% of patients (52% in non-survivors and 12% of survivors [p=<0.0001]). These data were corroborated by datasets from New York, which additionally identified that there were higher rates of intubation amongst patients who were obese.15,16 These datasets are large and provide detailed admission demographics but outcome data is limited due to short follow-up periods.
Cardiac biomarkers
Cardiac biomarkers (high sensitivity troponin T [hs-CRP] and NT pro-brain natriuretic peptide [NT pro-BNP]) have been investigated in observational studies in patients presenting with COVID-19 infection.6 Severe infection was associated with a higher rate of elevated troponin T and NT-pro-BNP in this cohort. Particularly interesting was that a higher troponin was correlated with both a higher NT-pro-BNP (R2 0.376, p=<0.001) and hs-CRP (R2 = 0.281, p<0.001). These results suggest increasing haemodynamic strain due to worsening infection as the aetiology for the increased troponin, and that this is associated with increased mortality. Whether the increasing haemodynamic strain is as a result of myocardial infiltration will be the topic of pathological studies to come. A potentially interesting analysis would be to plot a receiver operating characteristic against the delta in troponin in order to identify at what point increasing troponin is likely to result in a negative outcome.
ACE inhibitors and COVID-19 infection
ACE2 expression has been a topic of wide discussion since the emergence of the COVID-19 outbreak; specifically with regard to the use of angiotensin-converting enzyme (ACE) inhibitors and the possibility of upregulation of the ACE receptors, and thus the possibility of worsening the clinical course of the infection. This is of concern due to the fact that ACE inhibitor/angiotensin receptor blockers (ARBs) comprise the majority of first-line antihypertensive agents.17 This concept was highlighted from studies during the SARS CoV outbreak, where ACE inhibitors and ARBs were shown to promote an upregulation in messnger RNA for the ACE2 receptor.18 Additionally, the original mice studies involving SARS CoV infection have shown that subjects with downregulated myocardial ACE2 protein exhibit less viral infiltration of myocardial tissue.12
ACE2 receptors are upregulated in patients with diabetes,19 and this was hypothesised as a possible contributory factor to the increased mortality observed in patients with diabetes in the last SARS CoV outbreak.20,21 A recent study, however, has shown that ACE inhibitors do not worsen the clinical course of the infection, but in fact protect against worse outcome in patients with diabetes who get COVID-19 infection.22 The advice of all the major cardiac societies at this time is that patients on ACE inhibitors or ARBs should not stop treatment.
Hydroxychloroquine and azithromycin therapy
The rationale for the use of chloroquine was based on studies conducted on the SARS CoV outbreak which showed that, as the concentration of chloroquine increased in vitro, there was a reduction in SARS CoV infiltration in cells pre-treated 24 hours pre-infection.17 Additionally, cells treated with the agent three to five hours after infection showed lower levels of intracellular replication. It is thought that the effect of chloroquine is due to an inhibition of terminal-glycosylation of the ACE2 protein before it is released to the cell surface, which impedes virus entry to the cell.17 In cases of previously infected cells, it is believed that the chloroquine increases the endosomal pH where the virus resides within the cell, thus impeding intracellular reproduction mechanics.
Initial studies from China suggested that chloroquine acted beneficially in treating COVID-19 infection.18 One early in vitro study showed that chloroquine showed therapeutic activity in COVID-19 infection, as seen with the previous SARS CoV species.19 Here chloroquine exhibited activity in reducing the viral copy numbers via quantitative real-time RT-PCR, regardless of the timeframe of treatment (i.e. cells pre-treated with chloroquine, or treated after infection). Results from the first in man hydroxychloroquine study involved 62 patients and indicated benefit for 200 mg twice daily PO hydroxychloroquine.20 The outcome was time to clinical recovery and included body temperature recovery time (3.2 days control group vs. 2.2 days study group), cough resolution time and improvement in computed tomography findings (80.6% improved in the study arm vs. 54.8% improved in the control arm). A weakness of this study was that, despite apparent skew in the data (seen in the standard deviation data) and low numbers, parametric analysis was conducted (t-test and chi-squared). These factors may be of relevance when interpreting the potential benefits of hydroxychloroquine.
Up until recently, the largest study involved 181 patients over four centres in France who were admitted to hospital and required oxygen therapy.18 Here, investigators compared 84 patients who received hydroxychloroquine (400 mg per day) with 97 people who did not. The statistical methods used in this case were far more sophisticated and used a more robust outcome. The primary composite end point consisted of transfer to ICU within seven days of admission and the occurrence of death from any cause. The study found 20.5% patients in the hydroychloroquine group were transferred to the ICU or died within seven days, compared with 22.1% in the no-hydroxychloroquine group (RR 0.93, 95% CI 0.48–1.81). This study indicated that there was no statistical benefit in hydroxychloroquine in preventing ICU transfer or death.
Additionally, 20% of the hydroxychloroquine group were also commenced on azithromycin, which did not appear to confer additional benefit, and notably some of the patients had treatment withdrawn because of significant prolongation of the QT interval. This indicated a potential important safety concern over the widespread concomitant use of these two agents in COVID 19 infection. The use of azithromycin had gained traction since the publication of a small study involving 26 patients (6 of whom were treated with azithromycin) showing benefit in reducing time to virological remission.22
As we write this article, however, two large observational studies from New York were published which go to significant lengths to clarify the usefulness of hydroxychloroquine, and its combination with azithromicin in COVID-19 infection. The first involved 1,376 patients (811 patients receiving the drug and 565 not receiving the drug), and the end point was a composite of intubation or death.23 The patients receiving the drug were sicker, and so the crude unweighted hazard ratio (HR) was higher in the hydroxychloroquine group (HR 2.37, 95% CI 1.84–3.02). When this difference was corrected for with propensity-score, analysis showed no benefit in preventing intubation or death (HR 1.04, 95% CI 0.82–1.32). Notably, 486/811 (59.9%) of the patients in the hydroxychloroquine group were also given azithromycin, and this treatment was not associated with a difference in outcome (HR, 1.03; 95% CI, 0.81–1.31).
The second large observational study from New York served to further clarify any association between hydroxychloroquine and azithromycin treatment and outcome (inpatient mortality).24 This study, involving 1,438 patients admitted with COVID-19, also looked at a secondary (safety) outcome of cardiac arrest, arrhythmia or QT interval prolongation. Study participants were grouped by hydroxychloroquine and azithromycin therapy (n=735), hydroxychloroquine alone (n=271), azithromycin alone (n=211) and neither drug (n=221). Again, the patients receiving hydroxychloroquine and azithromycin were sicker (evidenced by lower oxygen saturations on presentation), and these patients had a higher crude mortality (25.7%, compared with all-comer mortality of 20.3%). When adjusted for baseline characteristics, there was no significant difference in outcome across the groups when compared with no treatment (hydroxychloroquine and azithromycin [HR 1.35, 95% CI 0.76–2.40], hydroxychloroquine alone [HR 1.08, 95%CI 0.63–1.85], or azithromycin alone [HR 0.56, 95%CI 0.26–1.21]). Regarding the secondary (safety) outcomes, there was a higher incidence of cardiac arrest in the hydroxychloroquine/azithromycin group (adjusted OR 2.13, 95%CI 1.12–4.05). QT prolongation was seen in higher frequencies in the hydroxychloroquine/azithromycin group (11%), compared with no treatment (5.9%)(p value between groups = 0.006).
QT prolongation
QT prolongation (figure 2) is of specific concern in the treatment of COVID-19 with use of hydroxychloroquine therapy. In a retrospective cohort analysis of 90 patients,25 seven patients (19%) who received hydroxychloroquine monotherapy developed prolonged QTc of 500 ms or more, and three patients (3%) had a change in QTc of 60 ms or more. Of these patients, 53 also had concomitant azithromycin therapy: 11 of 53 (21%) had prolonged QTc of 500 ms or more, and seven of 53 (13 %) had a change in QTc of 60 ms or more. The likelihood of prolonged QTc was greater in those who received concomitant loop diuretics (adjusted OR, 3.38 [95% CI, 1.03–11.08]) or had a baseline QTc of 450 ms or more (adjusted OR, 7.11 95% CI 1.75–28.87, p=0.008).
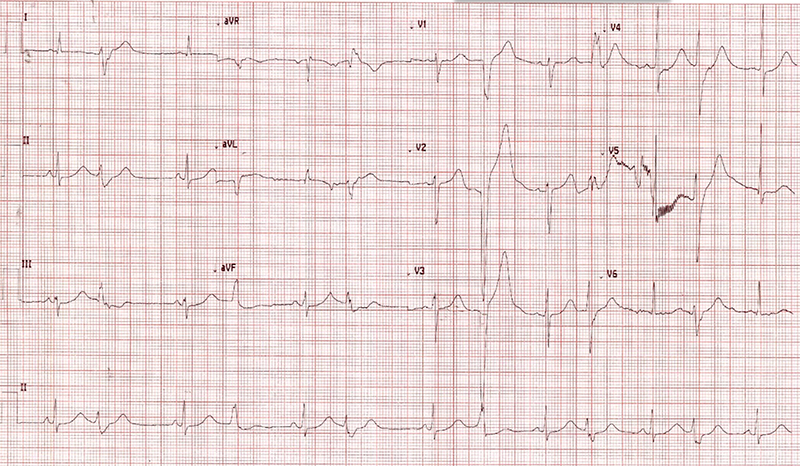
One study from Brazil enrolled patients to determine benefit from high-dose hydroxychloroquine 600 mg twice daily versus 450 mg once daily. All patients received concomitant azithromycin. The high-dose arm was discontinued early after randomising 41 patients to that group as there was a rate of mortality in excess of that allowed by design. Of the 11 patients who died in the study, seven (63.6%) were in the high-dosage group and four (36.4%) in the low-dosage group. QTc was observed to be greater than 500 ms more commonly in the high-dose group in this study (7 of 37 [18.9%] vs. 4 of 36 [11.1%]). Two of 37 patients (2.7%) in the high-dosage group, both with confirmed COVID-19, experienced ventricular tachycardia before death, without torsade de pointes.26
Remdesivir
Remdesivir, a nucleotide analogue, has been shown in vitro to be effective in reducing replication rate,27 and garnered interest following administration to a patient in the US which was published. The US Food and Drug Adminisitration (FDA) granted in May 2020 authorisation for its emergency use after preliminary results from a government sponsored study of over 1,000 patients, with a control group included, showed that the drug shortened the time to recovery by 31% or about four days on average, for hospitalised patients. Data from the compassionate release programme for severe infections (n=53) indicated an improvement in oxygen-support status in 68% of patients, with an overall mortality of 13% at a median follow-up of 18 days.20 It should be noted, however, that when studied in adults with severe COVID-19 in a randomised, double-blind, placebo-controlled, multi-centre trial fashion, and reported last month in The Lancet, the authors found no statistically significant clinical benefits, but reported that the numerical reduction in time to clinical improvement in those treated earlier required confirmation in larger studies.28 Editors’ note: see addendum for a preliminary report from another study with remdesivir since this article was written.
Smoking and COVID-19
The prevalence of smoking in China is 25.2%,29 and it was observed in COVID-19 analysis that the prevalence among those admitted to hospital was 12.6%.30 This led to a hypothesis that nicotine was protective against COVID-19 patients requiring hospitalisation.31
A French research group reported last month, after looking at 482 COVID-19 patients admitted to one hospital, that daily smokers had a very much lower probability of developing symptomatic or severe SARS-CoV-2 infection, suggesting that nicotine might adhere to cell receptors and prevent the virus from entering people’s cells. The media coverage has led the French government to give advice on avoiding the overconsumption of nicotine.32
Research in 1,099 patients from China, however, suggested that smokers were 1.4 times more likely to have severe symptoms of COVID-19, and almost 2.5 times more likely to be admitted to ICU, need mechanical ventilation, or die.30 A meta-analysis including 11,500 COVID-19 patients concluded that smoking was associated with almost a doubling of the risk of disease progression.33
The French researchers are planning to trial nicotine patches in the treatment of COVID-19. There are two possible confounders to the appearance of low incidence of smoking in this population. The first is that in China there is disparity in inpatient cost and insurance coverage,34 and this may have influenced certain socioeconomic groups when deciding to present to hospital. The second is that there is wide media reporting of a shortage of intensive care beds and peoplemay be less likely to report they are smokers if they think it is going to exclude them getting a bed in the ICU.
BCG and prevention of COVID-19 infection
The Bacillus Calmette–Guérin (BCG) has been known for a long time to employ significant modifications in the immune system (stimulating a trained immunity),35 which have been thought to possibly play a role in protection in COVID-19 infection. Specifically, with regard to a role in immunocompetency,36 the BCG has been shown to reduce the incidence of both all-cause pneumonia in the developing world and reduces viraemia in other similarly structured viruses to the COVID-19 virus.37–39 Whilst this concept is interesting, there are no data available for its use in coronaviruses (in either in vitro or in vivo conditions). Generally, BCG vaccination was quite common in Europe up to 20 years ago, and was part of the vaccination schedule in the UK from 1958 through to 2005.40 A recent study revealed a significant negative trend between countries that offer the BCG vaccine to the general population and the reported cases of COVID-19.41 Population-based studies across Europe relative to age and immunisation schedules will cast light on this issue. Additionally, there are a number of randomised-controlled studies in the pipeline that are awaited. In the meantime the World Health Organization have issued a statement advising against its use at this stage.42
Vitamin D deficiency
A recent paper identified a possible association between the incidence of COVID-19 and vitamin D levels.43 Vitamin D has been shown to be associated with effective immune function,44 specifically within the realm of antiviral processes.45 Authors investigated the relationship with average vitamin D level for each country and the corresponding death rate per capita from COVID-19. There was an observed correlation showing the lower the average vitamin D level, the higher the death rate by Spearman’s r (p=0.046). These results may very well be associative and serve as a marker for general nutrition levels, but is nonetheless hypothesis generating, and patient-level study would be useful to develop this concept further.
The paediatric population and ‘Kawasaki disease’
The paediatric population appeared to have been relatively spared from COVID-19 infection as reflected by national databases from the UK and the US, with rates of 2% and 1% of cases, respectively.46,47 The true incidence of COVID-19 in the paediatric population is difficult to determine,48 as observational studies require either a close contact or a child with symptomatology in order to be identified, and a large proportion of asymptomatic individuals are never identified. Even within these limitations, of those identified, the majority of paediatric patients experience either no symptoms, or mild/moderate illness (94.1% of cases).
There were, however, initial reports of clusters of a Kawasaki-like illness from Bergamo in Italy during the height of the COVID-19 outbreak there.49 Kawasaki disease is an acute vasculitis seen in young children with fever, a rash, conjunctival injection, and lymphadenopathy. In this cohort, the children were older than those affected before the COVID-19 outbreak (7.5 years vs.3 years, p=0.00035), had a higher incidence of abnormal echo (60% vs. 10%, p=0.0089) and had a higher rate of both Kawasaki disease shock syndrome and macrophage activation syndrome (both 50% vs. 0%, p=0.021). Further clusters have now arisen in areas experiencing high rates of COVID-19 infection, such as London.50 Authors describe a further case series of emerging atypical Kawasaki disease exhibiting high incidences of high-grade inflammation and left ventricular dysfunction. In response to this, the Royal College of Paediatrics have issued a guidance document for this disorder which has been labelled as ‘paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS)’.51 Last weekend, Governor Andrew Cuomo in New York reported an outbreak of 75 cases and three deaths from this (emerging) disease and more-recently defined diagnostic criteria and announced it as a notifiable disease in New York.52
Summary
We know from previous similar coronaviruses that myocardial tissue is infiltrated; and this is particularly the case in the most severe infections. This is reflected in a significant incidence of heart failure in patients with COVID-19 infection, and aggressive investigation (echo, BNP, troponins etc) and treatment is very much part of the multidisciplinary in-hospital approach to treatment of the COVID-19 patient.
Hydroxychloroquine as a credible treatment for COVID-19, either alone or with azithromycin, does not look encouraging, with the publication of two large well-designed trials in the last week showing no clear benefit. This is in parallel with the emerging safety data indicating that the combination of hydroxychloroquine and azithromycin significantly predisposes patients to ventricular arrhythmia for no benefit. Whatever remaining enthusiasm exists for using, particularly the combination of, these agents, they should NOT be used outside the ECG-monitored hospital setting. Although remdesivir was ineffective in treating Ebola disease, it seems to have potential beneficial effects in modifying the severity of the in-hospital COVID-19 course, and further studies will hopefully more firmly identify its place in the in-hospital treatment regime.
Finally, physicians looking after the paediatric population may have a significant foe to deal with in the coming months in the form of paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) (and indeed commentators may need to be more cautious in informing the public that children have no problem with the virus). This immune-mediated disorder does not appear to have a fixed time-course based on the data available. Clusters are showing emergence of this illness at various time-points of interaction with COVID-19 infection, from the acute phase where the patients are shedding the virus to late phase wherein patients have seroconverted to an IgG response. The coming months are likely to shed valuable light on this mysterious entity.
Addendum
As we go to press, a multicentre, randomised, controlled study involving 1,063 patients has found that remdesivir shortened the median time to clinical recovery compared with placebo (11 days vs. 15 days, respectively; rate ratio for recovery, 1.32; 95%CI 1.12–1.55; p<0.001). Additionally, there was a numerical improvement in mortality at 14 days between the groups (7.1% vs. 11.9%, respectively, HR 0.70, 95%CI 0.47–1.04). More long-term follow-up (such as 30 day mortality is pending for this group and will provide further insight to the usefulness of this agent.53
In response to the emerging data from large hydroxychloroquine trials, the WHO has suspended the hydroxychloroquine arm of the ongoing SOLIDARITY trial.54 The validity of the data, from which the WHO ultimately made their decision, is being reviewed following significant concerns expressed by members of the scientific community.
Key messages
- Cardiovascular risk factors significantly predispose patients to worse outcomes with COVID-19 infection
- ACE inhibitors do not appear to worsen the clinical course of the infection
- It is unclear what effect smoking, BCG vaccination and vitamin D deficiency may have on COVID-19
- Clusters of an atypical Kawasaki disease appear to be emerging in children in areas experiencing high rates of COVID-19 infection
Conflicts of interest
None declared.
Funding
None required.
References
1. Wuhan Municipal Health Commission. Cardiovascular complications of coronavirus. Report of clustering pneumonia of unknown etiology in Wuhan City. Published 31st December 2019.
2. World Health Organization. Coronavirus disease (COVID-19) Situation Report – 118. Data as received by WHO from national authorities by 10:00 CEST, 17th May 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (last accessed 27th May 2020)
3. Zhu N, Zhang D, Wang W, et al. for the China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. https://doi.org/10.1056/NEJMoa2001017
4. Zhang T, Wu Q, Zhang Z (19 March 2020). Probable pangolin origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Current Biology 2020;30:1346–51.e2. https://doi.org/10.1016/j.cub.2020.03.022
5. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020;63(3):457–60. https://doi.org/10.1007/s11427-020-1637-5
6. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; published online 27th March 2020. https://doi.org/10.1001/jamacardio.2020.1017
7. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450–4. https://doi.org/10.1038/nature02145
8. Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci 2004;25:291–4. https://doi.org/10.1016/j.tips.2004.04.001
9. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11:875–9. https://doi.org/10.1038/nm1267
10. Crackower MA, Sarao R, Oudit GY, et al. Angiotensin converting enzyme 2 is an essential regulator of heart function. Nature 2002;417:822–8. https://doi.org/10.1038/nature00786
11. Oudit GY, Crackower MA, Backx PH, Penninger JM. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med 2003;13:93–101. https://doi.org/10.1016/S1050-1738(02)00233-5
12. Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 2009;39(7):618–25. https://doi.org/10.1111/j.1365-2362.2009.02153.x
13. Haga S, Yamamoto N, Nakai-Murakami C, et al. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci USA 2008;105:7809–14. https://doi.org/10.1073/pnas.0711241105
14. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. https://doi.org/10.1016/S0140-6736(20)30566-3
15. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020; published online 17th April 2020. https://doi.org/10.1056/NEJMc2010419
16. Richardson S, Hirsch JS, Narasimhan M, et al; and the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; published online 22nd April 2020. https://doi.org/10.1001/jama.2020.6775
17. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology Journal 2005;2:article number 69. https://doi.org/10.1186/1743-422X-2-69
18. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioSci Trends 2020; published online 29th February 2020. https://doi.org/10.5582/bst.2020.01047
19. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020. https://doi.org/10.1038/s41422-020-0282-0
20. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv 2020. https://doi.org/10.1101/2020.03.22.20040758
21. Mahevas M, Tran VT, Roumier M, et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. medRxiv 2020. https://doi.org/10.1101/2020.04.10.20060699
22. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020;published online 20th March 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949
23. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020; published online 7th May 2020. https://doi.org/10.1056/NEJMoa2012410
24. Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA 2020; published online 11th May 2020. https://doi.org/10.1001/jama.2020.8630
25. Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; e-publication online 1st May 2020. https://doi.org/10.1001/jamacardio.2020.1834
26. Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 2020;3(4):e208857. https://doi.org/10.1001/jamanetworkopen.2020.8857
27. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19. N Engl J Med 2020. Published online 10th April 2020. https://doi.org/10.1056/NEJMoa2007016
28. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395(10236):P1569–78. https://doi.org/10.1016/S0140-6736(20)31022-9
29. Wang M, Luo X, Xu S, et al. Trends in smoking prevalence and implication for chronic diseases in China: serial national cross-sectional surveys from 2003 to 2013. Lancet Respir Med 2018;7:35–45. https://doi.org/10.1016/S2213-2600(18)30432-6
30. Guan W, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. https://doi.org/10.1056/NEJMoa2002032
31. Farsalinosa K, Niaura R, Le Houezec J. Nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol Rep 2020; published online ahead of print 30th April 2020. https://doi.org/10.1016/j.toxrep.2020.04.012
32. Miyara M, Tubach F, Pourcher V, et al. Low incidence of daily active tobacco smoking in patients with symptomatic COVID-19. Qeios ID: WPP19W.3. Published online 21st April 2020. https://doi.org/10.32388/WPP19W.3
33. He, A.J., Wu, S. Towards universal health coverage via social health insurance in China: systemic fragmentation, reform Imperatives, and policy alternatives. Appl Health Econ Health Policy 2017;15:707–16. https://doi.org/10.1007/s40258-016-0254-1
34. Patanavanich R, Glantz SA. Smoking Is associated with COVID-19 progression: a meta-analysis. Nicotine & Tobacco Research 2020;ntaa082. Published online 11th May 2020. https://doi.org/10.1093/ntr/ntaa082
35. Netea MG, Dominguez-Andres J, Barreiro LB, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol 2020. Published online 4th March 2020. https://doi.org/10.1038/s41577-020-0285-6
36. Pollard AJ, Finn A, Curtis N. Non-specific effects of vaccines: plausible and potentially important, but implications uncertain. Arch Dis Child 2017;102: 1077–81. https://doi.org/10.1136/archdischild-2015-310282
37. Biering-Sorensen S, Aaby P, Lund N et al. Early BCG-Denmark and neonatal mortality among infants weighing <2500 g: a randomized controlled trial. Clin Infect Dis 2017;65:1183–90. https://doi.org/10.1093/cid/cix525
38. Nemes E, Geldenhuys H, Rozot V, et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med 2018;379:138–49. https://doi.org/10.1056/NEJMoa1714021
39. Arts RJW, Moorlag S, Novakovic B, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 2018;23:89–100. https://doi.org/10.1016/j.chom.2017.12.010
40. Zwerling A, Behr MA, Verma A, et al. The BCG world atlas: a database of global BCG vaccination policies and practices. PLoS Med 2011;8(3):e1001012. Published online 22nd Mar 2011. https://doi.org/10.1371/journal.pmed.1001012
41. Mariita RM and Mulila JM. A study on the relationship between BCG vaccination and Covid-19 prevalence: Do other confounders warrant investigation? MedRxiv 2020. Published online 11th May 2020. https://doi.org/10.1101/2020.05.06.2009313842
42. The World Health Organization. Bacille Calmette-Guérin (BCG) vaccination and COVID-19 Scientific Brief. Published 12th April 2020. https://www.who.int/news-room/commentaries/detail/bacille-calmette-gu%C3%A9rin-(bcg)-vaccination-and-covid-19 (last accessed 27th May 2020)
43. Laird E, Rhodes J, Kenny RA. Vitamin D and Inflammation: potential Implications for severity of COVID-19. Ir Med J 2020;113(5):P81. http://imj.ie/wp-content/uploads/2020/05/Vitamin-D-and-Inflammation-Potential-Implications-for-Severity-of-Covid-19.pdf (last accessed 27th May 2020)
44. Vanherwegen AS, Gysemans C, Mathieu C. Regulation of immune function by vitamin D and its use in diseases of Immunity. Endocrinol Metab Clin North Am 2017;46(4):1061–94. https://doi.org/10.1016/j.ecl.2017.07.010
45. Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol 2011;50(3):194–200. https://doi.org/10.1016/j.jcv.2010.12.006
46. Docherty AB, Harrison EM, Green CA, et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO clinical characterisation protocol. medRxiv 2020. Published online 28th April 2020. https://doi.org/10.1101/2020.04.23.20076042
47. Bialek S, Gierke R, Hughes M. Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep 2020;69:422–6. https://doi.org/10.15585/mmwr.mm6914e4
48. Dong Y, Mo X, Hu Y et al. Epidemiological characteristics of 2143 paediatric patients with 2019 coronavirus disease in China. Pediatrics 2020. https://doi.org/10.1542/peds.2020-0702
49. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020. Published online 13th May 2020. https://doi.org/10.1016/S0140-6736(20)31103-X
50. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020. Published online 7th May 2020. https://doi.org/10.1016/S0140-6736(20)31094-1
51. Royal College of Paediatrics and Child Health. Guidance—Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (1 May 2020). https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19 (last accessed 27th May 2020)
52. Cuomo A, Zucker HA, Dreslin S for New York State Department of Health. Health Advisory: Pediatric multi-system inflammatory syndrome temporally associated with COVID-19 interim case definition in New York State. Published online 13th May 2020. https://health.ny.gov/press/releases/2020/docs/2020-05-13_health_advisory.pdf (last accessed 27th May 2020)
53. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — preliminary report. N Engl J Med 2020; published online 22nd May 2020. https://doi.org/10.1056/NEJMoa2007764
54. World Health Organisation. WHO Director-General’s opening remarks at the media briefing on COVID-19, 25th May 2020 https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—25-may-2020 (last accessed 3rd June 2020).
