Stroke prophylaxis in atrial fibrillation is an important consideration in patients with cancer. However, there is little consensus on the choice of anticoagulation, due to the numerous difficulties associated with active cancer. Direct oral anticoagulants (DOACs) have been shown to be a promising option. Here, we conduct a simple cross-sectional analysis of 29 cancer patients receiving DOACs for stroke prophylaxis in atrial fibrillation at a tertiary-care institution in London. Our study demonstrates an encouraging efficacy and safety profile of DOACs used in this setting. We conclude by suggesting that, while DOACs may be useful, anticoagulation in cancer patients should continue to be individualised.
Background
Atrial fibrillation (AF) occurs at an increased frequency in patients with cancer, however, the link between cancer and AF is not fully understood.1,2 Stroke prophylaxis with anticoagulation is important; however, this can prove challenging in the setting of active malignancy, which predisposes to an increased risk of haemorrhage, as well as thrombosis.3 Traditionally, low molecular weight heparin (LMWH) and warfarin have been used in this setting.
There remains limited evidence for direct oral anticoagulants (DOACs) in cancer patients for stroke prophylaxis in AF (SPAF). However, small observational studies have demonstrated comparable safety and efficacy profiles against warfarin and LMWH.4,5 Compared with warfarin, Shah et al. demonstrated similar bleeding rates in cancer patients with AF taking rivaroxaban/dabigatran, and lower bleeding rates in patients taking apixaban, with no significant difference in ischaemic stroke rates among anticoagulant users.4 The ENGAGE AF-TIMI (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction) trial found preserved efficacy and safety of edoxaban compared with warfarin.5 Other retrospective studies have found fewer bleeding events and preservation of efficacy with DOACs compared with LMWH.
With a lack of consensus regarding optimal anticoagulation in cancer patients with AF, there remains highly variable clinical practice. Here, we conduct a cross-sectional study on DOAC prescribing in AF and cancer at a single tertiary-care institution.
Method
A population of patients with concomitant AF and active cancer who were prescribed DOACs for SPAF were identified through monitoring of all DOAC prescriptions over six months at St. Bartholomew’s Hospital. A cross-sectional analysis was then conducted, with a particular focus on stroke/transient ischaemic attack (TIA) and bleeding episodes. Bleeding episodes were divided into major and non-major, as per the International Society on Thrombosis and Haemostasis (ISTH).
Results
There were 29 patients with cancer diagnoses who received DOACs for SPAF. The median age was 78 years (interquartile range [IQR] 69–82 years) and there was a male preponderance (83%). AF developed following cancer diagnosis in 59% of patients, and 21% of patients had an unknown onset date. The mean duration of DOAC therapy was 10.9 months at the time of cross-sectional analysis. DOACs used included apixaban (52%), edoxaban (24%) and rivaroxaban (24%). The median CHA2DS2-VASc and HAS-BLED scores were 3 and 2, respectively.
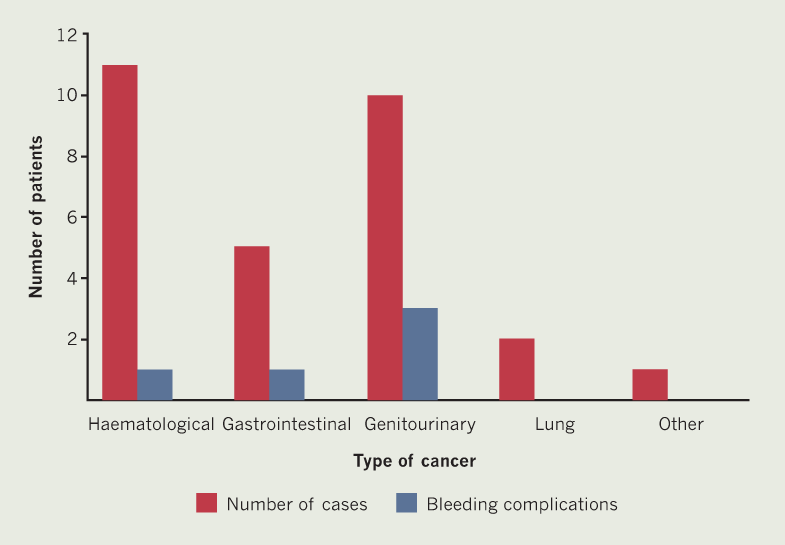
There were no embolic strokes/TIAs identified in any patient following commencement of DOAC (figure 1), including in the 25 high-risk patients (CHA2DS2-VASc score ≥2). There were no major bleeding events. Five patients suffered from non-major bleeding, four of whom had gastrointestinal or genitourinary cancer. Nine patients were high-risk for bleeding (HAS-BLED score ≥3), none of whom experienced bleeding events.
Discussion
Our findings show that DOACs for SPAF in cancer can be effective, as evidenced by a lack of both thromboembolic events and major bleeding episodes, including in high-risk patients. Furthermore, non-major bleeding events occurred in patients predominantly with gastrointestinal/genitourinary cancers, which are both associated with a higher bleeding risk.
This was a small study with 29 patients recruited from a single institution. Due to the particular expertise of the tertiary-care centre, the types of cancers included were restricted (there was an over-representation of haematological cancers). The mean duration of DOAC treatment was also quite short (10.9 months), therefore, limiting longer-term evaluation. However, while we had limited numbers of patients with limited follow-up, it is likely our local data are reflective of wider UK practice in tertiary cardiology and cancer referral centres.
Cancer is a challenging setting in which to formulate anticoagulation strategies. Multiple observational studies have demonstrated a promising role for DOACs in SPAF and cancer, however, these are difficult to interpret because of exclusion and heterogeneity across cancers. We believe our local data review adds to this growing collection of evidence, although the way forward will also require directed trials in particular patient subpopulations. Importantly, due to the many difficulties associated with active cancer, we believe anticoagulation still needs to be individualised to provide optimal outcomes, in accordance with the recent guidance issued by the International Society on Thrombosis and Haemostasis (ISTH),1 taking into account cancer type, stroke and bleeding risk, comorbidities, and ongoing cancer-directed treatment.
Key messages
- Direct oral anticoagulants (DOACs) may be a useful option for stroke prophylaxis in atrial fibrillation (AF) in the context of active cancer, with preserved efficacy and safety compared with low molecular weight heparin (LMWH) and warfarin
- Specialist cardio-oncology centres and registries can fill data gaps, however, more directed trials are required to establish optimal anticoagulation approaches in active cancer
- Due to the many challenges associated with cancer, anticoagulation must remain an individualised treatment
Conflicts of interest
None declared.
Funding
None.
Study approval
Not required due to the anonymised, retrospective nature of this study.
References
1. Delluc A, Wang T, Yap E et al. Anticoagulation of cancer patients with non-valvular atrial fibrillation receiving chemotherapy: guidance from the SSC of the ISTH. J Throm Haemostasis 2019;17:1247–52. https://doi.org/10.1111/jth.14478
2. Mosarla R, Vaduganathan M, Qamar A et al. Anticoagulation strategies in patients with cancer. J Am Coll Cardiol 2019;73:1336–49. https://doi.org/10.1016/j.jacc.2019.01.017
3. O’Neal W, Claxton J, Sandesera P et al. Provider specialty, anticoagulation, and stroke risk in patients with atrial fibrillation and cancer. J Am Coll Cardiol 2018;72:1913–22. https://doi.org/10.1016/j.jacc.2018.07.077
4. Shah S, Norby F, Datta Y et al. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv 2018;2:200–09. https://doi.org/10.1182/bloodadvances.2017010694
5. Fanola C, Ruff C, Murphy S et al. Efficacy and safety of edoxaban in patients with active malignancy and atrial fibrillation: an analysis of the ENGAGE AF-TIMI 48 trial. J Am Heart Assoc 2018;7:e008987. https://doi.org/10.1161/JAHA.118.008987
