This review focuses on the role of CytoSorb® (CytoSorbents Corporation, Monmouth Junction, New Jersey, USA), a technology for purifying extracorporeal blood. The technology is designed for several indications to prevent bleeding complications during on-pump cardiac surgery, including removal of the antiplatelet agent, ticagrelor, and the oral anticoagulant, rivaroxaban, from the blood. Recent clinical studies are briefly reviewed.
Introduction
Bleeding post cardiac surgery carries significant patient mortality and morbidity including resternotomy, increased hospital stays, and blood product transfusion.1,2 Bleeding is more severe in patients who are taking pre-operative antiplatelet or anticoagulation medications that have not been stopped sufficiently in advance to minimise such risks. Current guidelines across Europe recommend stopping such agents at least two to five days prior to surgery; they also recommend delaying urgent surgery so that the risk of perioperative bleeding is minimised.3 However, this is not always possible in the setting of urgent and emergency cases, as the risk of adversity delaying surgery outweighs the risk of bleeding.
Some agents, such as warfarin, can be reversed using vitamin K, or bleeding risk can be minimised e.g. with dabigatran, a direct thrombin inhibitor, by using an antidote (reversal agent) idarucizumab.3 For other direct oral anticoagulants (DOACs), namely factor Xa inhibitors rivaroxaban and apixaban, andexanet alfa is an approved antidote when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding4, but not to prevent it. In the majority of cases, blood products including fresh frozen plasma, cryoprecipitate and platelets, are used to minimise the effect of such agents on bleeding during the perioperative period. It is imperative to understand that not only patients on anticoagulation or antiplatelet medications have a higher risk of bleeding – in infective endocarditis, for example, patients are at high risk due to the systemic coagulopathy associated with sepsis.
Despite using the above measures, bleeding remains a critical issue in emergency cases and high-risk patients, and very often contributes to the higher rate of perioperative complications and poorer outcomes in these patients.5 It is not only bleeding that can significantly impact the outcomes but the accompanying inflammatory and cytokine response can fulminate the coagulopathic process further affecting outcomes during and post cardiac surgery.
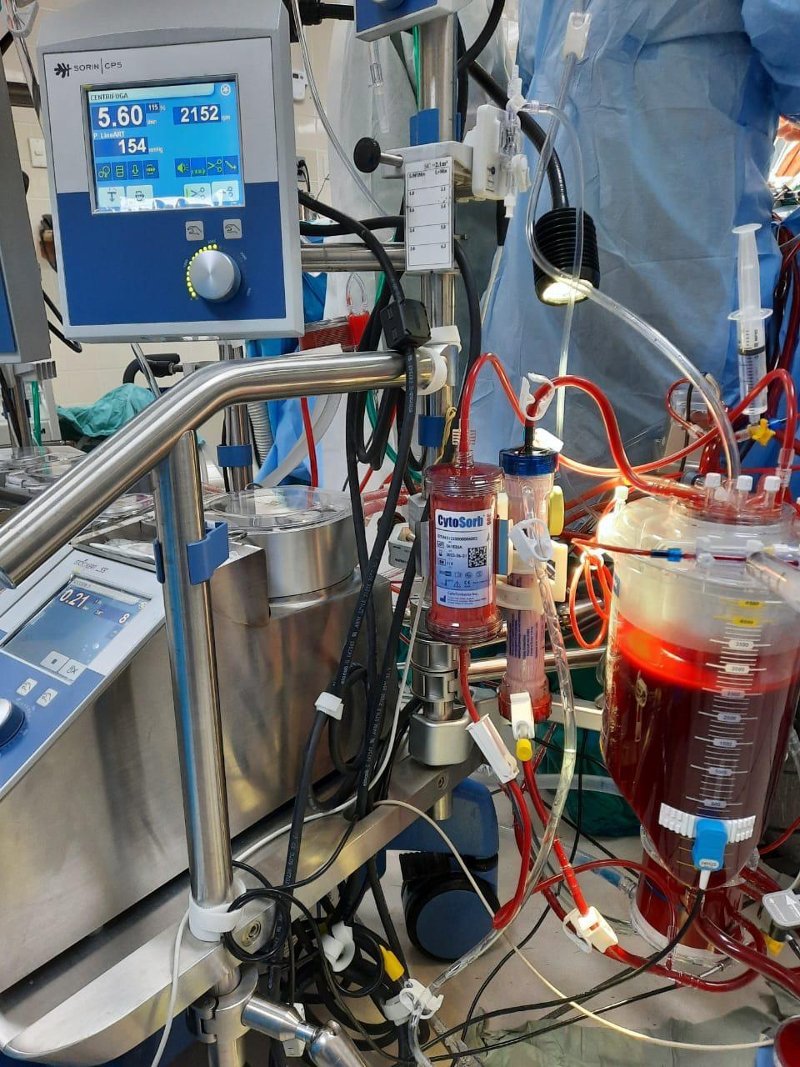
What is CytoSorb®?
The CytoSorb® system is used in patients who require urgent or emergency cardiac surgery, including for acute cardiovascular events, acute deterioration, or a failed percutaneous coronary intervention. CytoSorb® consists of a 300 ml cartridge containing small absorbent polymer beads that permit the removal of hydrophobic substances. The sorbent cartridge can be stored for up to 36 months. The CytoSorb® CE label now also includes intraoperative removal of ticagrelor and rivaroxaban during cardiopulmonary bypass surgery. The technology is used by cardiothoracic surgeons, cardiac anaesthetists, perfusionists, and specialised nurses. It is used in tertiary care during cardiac surgery.
The manufacturer states that CytoSorb® can be integrated into the extracorporeal circuit (see right) by a perfusionist or specialised nurse and only minimal training is needed.
Haemoadsorption with CytoSorb® – the rationale
A key factor driving the coagulopathic status is the development of a cytokine storm during the perioperative period. This is due to the release of different inflammatory mediators during the cardiopulmonary bypass (CPB) process, such as interleukin (IL) 6, 8, 10, and tumour necrosis factor (TNF)-α among others, or as a part of the clinical picture of sepsis in the perioperative period. Systemic inflammation results in activation of coagulation, due to tissue factor-mediated thrombin generation, downregulation of physiological anticoagulant mechanisms, and inhibition of fibrinolysis.6
Haemoadsorption is a technique in which selective molecules can be removed during CPB or extracorporeal membrane oxygenation (ECMO).7 Through this technique, CytoSorb® eliminates the key contributors to the cytokine storm (IL-6, IL-8, IL-10; TNF-α, damage associated molecular patterns [DAMPs] such as C3a, C5a, S100; and pathogen associated molecular patterns [PAMPs] such as certain Gram-positive bacteria toxins), together with myoglobin and some metabolites (such as bilirubin, ammonia and bile acids). Furthermore, CytoSorb® adsorbs several drugs such as ticagrelor and rivaroxaban while preserving the coagulation factors and proteins.8
CytoSorb® is a recently launched product in which blood purification is utilised through the extracorporeal system with a very large surface area of around 45,000 m2 when compared to the filtration techniques in haemodialysis. The use of this asset is reported to help in eliminating the inflammatory mediators and antithrombotics to minimise the risk of perioperative complications including bleeding in patients using DOAC or antiplatelet agents.9 This plays a crucial role during emergency surgery in anticoagulated patients and in patients with infective endocarditis, who are at the highest risk of developing bleeding due to destructive maladaptive systemic inflammatory response (SIRS).10,11 The application of CytoSorb® is not limited to CPB, as it can be used during ECMO, dialysis or even haemoperfusion as part of an extracorporeal circulation circuit. Further future opportunities for using CytoSorb® include high-risk cardiac surgery patients, mechanical circulatory support devices related cases, and heart transplant cohorts.
Clinical and experimental data
CytoSorb® has been utilised in different critically ill patients including those with sepsis.12 In 2017, an in vitro study by Angheloiu et al. reported the successful use of haemoadsorption for the removal of ticagrelor, a P2Y12 receptor antagonist antiplatelet agent, and recommended its use during a clinical scenario involving a patient loaded with ticagrelor, undergoing cardiac catheterisation and then referred immediately to open heart surgery.8 In 2020, a study by Bjørnstad et al. emphasised that oral anticoagulation use is associated with increased mortality rates in patients with acute aortic diseases requiring emergency intervention.13 Following this, several – although limited – studies have reported outcomes utilising CytoSorb® in patients needing urgent or emergency cardiac surgery. Bradic et al.14 reported results in 35 patients between 2016–2019: in 63% of the patients CytoSorb®-use was associated with shorter operative times, lower postoperative drain outputs, lower blood transfusions, lower resternotomy rates and shorter intensive care unit stay. Similar outcomes were supported by an earlier and larger German study, which enrolled 55 patients that needed emergency cardiac surgery.15
Economically, the use of CytoSorb® is claimed to save costs when utilised in the correct cohort of patients. It is reported to save GBP 3,941 per patient who had ticagrelor or the DOAC, rivaroxaban, before emergency cardiac surgery.16 Several ongoing studies evaluating both drug removal and drug reversal strategies will contribute important new information on the ability to reduce the bleeding risk in patients on ticagrelor undergoing surgery (table 1). Nevertheless, a recent propensity score matched retrospective study in septic patients by Brouwer et al. showed that a combination of CytoSorb® with continuous renal replacement therapy (CRRT) resulted in a lower mortality rate than using CRRT alone (p=0.038) in septic shock patients with severe acute kidney injury.
Table 1. Summary of ongoing clinical trials
| Trial | Cohort | Estimated completion | Summary of study aims |
|---|---|---|---|
| TISORB (Ticagrelor CytoSorb® Hemoadsorption) NCT04131959 |
Prospective, open, multi-centre, single arm study N=30 |
August 2021 | To report that the CytoSorb® device removes ticagrelor from blood during surgery in patients who need emergency cardiac surgery and therefore decreases the risk for surgical bleeding from ticagrelor |
| CyTation (Ticagrelor Removal Study Using CytoSorb® 300 mL Device During CPB in Patients Undergoing Emergent Cardiothoracic Surgery) NCT04625764 |
A prospective, open, multi-centre, single-arm study N=30 |
January 2022 | To demonstrate intraoperative removal of ticagrelor by CytoSorb® haemadsorption in patients on ticagrelor undergoing emergent cardiac surgery requiring cardiopulmonary bypass |
| REVERSE-IT (Bentracimab [PB2452] in ticagrelor-treated patients with uncontrolled major or life-threatening bleeding or requiring urgent surgery or invasive procedure) NCT04286438 |
A phase 3, multi-centre, open-label, single-arm study N=200 |
December 2023 | To assess the reversal of the antiplatelet effects of ticagrelor with bentracimab (PB2452) in patients who present with uncontrolled major or life-threatening bleeding or who require urgent surgery or invasive procedures. |
The outcomes from the above-published literature have prompted the National Institute for Health and Care Excellence (NICE) to develop a medtech innovation briefing (MIB) to consider CytoSorb® as the first device to be demonstrated as safe and effective in removing ticagrelor from the circulation. According to the NICE MIB,17 CytoSorb® provides significant advantages in reducing the risk of bleeding in emergency cardiac surgery in patients treated with ticagrelor and lowers the utility of bleeding resources. CytoSorb® can, therefore, also reduce inhospital stay for urgent cases awaiting cardiac surgery.
Summary
While evidence is currently limited, there appears to be a trend that the novel approach of CytoSorb® haemoadsorption can be an effective tool to minimise bleeding risk in emergency cardiac surgery of patients treated with ticagrelor or rivaroxaban, and that CytoSorb® can contribute to shorter inhospital stays among urgent preoperative cases with a significant associated cost saving. Evidence however, is limited to case series and observational studies. Results from larger sample size and randomised trials are therefore keenly awaited in building a recommendation for regular use of CytoSorb® in on-pump cardiac surgery.
Key messages
- CytoSorb® is an established technology for extracorporeal blood purification
- Studies show it is effective for removing ticagrelor and rivaroxaban from blood
- Further studies are awaited to promote its routine use in on-pump cardiac surgery
Further information
The webinar ‘CytoSorb® adsorption for reducing risk of bleeding during cardiac surgery in ticagrelor/rivaroxaban treated patients’ on which this review is based can be accessed at: www.cyto.news/webinar-tica-riva
Conflicts of interest and funding
The BJC commissioned AH and AB who received an honorarium for writing this review. The review was sponsored by CytoSorbents Europe (see sponsorship statement).
References
1. Whitlock R, Crowther MA, Ng HJ. Bleeding in cardiac surgery: its prevention and treatment – an evidence-based review. Crit Care Clin 2005;21(3):589–610. https://doi.org/10.1016/j.ccc.2005.04.003
2. Petrou A, Tzimas P, Siminelakis S. Massive bleeding in cardiac surgery. Definitions, predictors and challenges. Hippokratia 2016;20(3):179–86.
3. Sousa-Uva M, Head SJ, Milojevic M, et al. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg 2018;53(1):5–33. https://doi.org/10.1093/ejcts/ezx314
4. European Medicines Agency. Ondexxya – Summary of Product Characteristics 2019. https://www.ema.europa.eu/en/documents/product-information/ondexxya-epar-product-information_en.pdf [last accessed 26th April 2021]
5. Al-Attar N, Johnston S, Jamous N et al. Impact of bleeding complications on length of stay and critical care utilization in cardiac surgery patients in England. J Cardiothorac Surg 2019;14:64. https://doi.org/10.1186/s13019-019-0881-3
6. Levi M, Keller TT, van Gorp E, ten Cate H. Infection and inflammation and the coagulation system. Cardiovascular Res 2003;60(1):26–39.
7. Cantaluppi V, Marengo M, Peng Z-Y, et al. Chapter 94 – Blood purification for sepsis. In: Ronco C, Kellum JA, Bellomo R, Ricci Z (eds). Critical care nephrology (Third Edition). Elsevier, 2019;548-552.e1. https://doi.org/10.1016/B978-0-323-44942-7.00094-7
8. Angheloiu GO, Gugiu GB, Ruse C, et al. Ticagrelor removal from human blood. JACC Basic Transl Sci 2017;2:135–45. https://doi.org/10.1016/j.jacbts.2017.01.007
9. Ankawi G, Xie Y, Yang B, et al. What have we learned about the use of cytosorb adsorption columns? Blood Purif 2019;48:196–202. https://doi.org/10.1159/000500013
10. Lees NJ, Rosenberg A, Hurtado-Doce AI, et al. Combination of ECMO and cytokine adsorption therapy for severe sepsis with cardiogenic shock and ARDS due to Panton-Valentine leukocidin-positive Staphylococcus aureus pneumonia and H1N1. J Artif Organs 2016;19:399–402. https://doi.org/10.1007/s10047-016-0915-8
11. Gruda MC, Ruggeberg KG, O’Sullivan P, et al. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PLoS One 2018;13(1):e0191676. https://doi.org/10.1371/journal.pone.0191676
12. Brouwer WP, Duran S, Kuijper M, Ince C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study. Crit Care 2019;23:317. https://doi.org/10.1186/s13054-019-2588-1
13. Bjørnstad JL, Khan AM, Røed-Undlien H, et al. Operative survival in patients with acute aortic disease in the era of newer oral anticoagulants. Open Heart 2020;7:e001278. https://doi.org/10.1136/openhrt-2020-001278
14. Bradic N, Rudez I, Neuberg M, Povsic-Cevra Z. Adsorption of DOAC medications with Cytosorb filter in patients undergoing cardiac surgery. Perfusion 2020;35:93–282
15. Hassan K, Kannmacher J, Wohlmuth P, Budde U, Schmoeckel M, Geidel S. Cytosorb adsorption during emergency cardiac operations in patients at high risk of bleeding. Ann Thorac Surg 2019;108:45–51. https://doi.org/10.1016/j.athoracsur.2018.12.032
16. Javanbakht M, Trevor M, Rezaei Hemami M, et al. Ticagrelor removal by CytoSorb® in patients requiring emergent or urgent cardiac surgery: a UK-based cost-utility analysis. Pharmacoecon Open 2020;4:307–19. https://doi.org/10.1007/s41669-019-00183-w
17. National Institute for Health and Care Excellence. CytoSorb for reducing risk of bleeding during cardiac surgery. Medtech innovation briefing (MIB 249]. Published 2nd February 2021. London: NICE, 2021. https://www.nice.org.uk/advice/mib249 [last accessed 5th May 2021]
