A retrospective study of 322 patient experiences of post-operative pain, short term and long term, following a cardiac implantable electronic device (CIED) procedure. Pain from pacemaker and ICD (implantable cardioverter-defibrillator) implant surgery remains a problem both in terms of severity and longevity. There is a subset of patients receiving implants that have severe pain that may be of a long duration. Patient advice needs to be appropriate to these findings.
This study illustrates a need for better pain management by physicians, support, and realistic communication with their patients.
Introduction

Indications for the use of pacemakers and implantable cardioverter-defibrillators (ICDs) have become more defined over time, resulting in many more patients receiving these devices.1 Cardiac implantable electronic device (CIED) procedure is the term used to encompass pacemaker and ICD implant surgery.
As of 2016, it was estimated that there were about 1.14 million pacemakers globally. By the year 2023, that number is expected to increase to 1.43 million units.2 Many nations are now keeping records of the implant complication rate for pacemakers and ICDs.3-6 As in any surgical procedure, a complication rate is to be expected from device implantation.7-9 Unfortunately, complication definition is a medical categorisation. Pain can be mentioned as a complication: “Minor wound pain is expected after device implantation, almost always controlled with simple analgesia”.10
Post-operative implant pain has been acknowledged and managed more robustly.11-13 It was concluded by Lee et al.11 that “Post-operative opioid prescription rate after CIED procedures was 20.2%, with most patients being opioid naïve. Among opioid naïve patients who received opioids, 9.4% had subsequent opioid refills. This finding suggests that peri-operative pain management in CIED procedures warrants meticulous attention.” Furthermore, patient advice may still be inadequate with regard to pain expectation. The current UK National Health Service (NHS) information to patients states, “You may feel some pain or discomfort during the first 48 hours and will be given pain-relieving medication”.14
Method
A retrospective study of 322 patient experiences of post-operative pain, short term and long term (<1 week to >5 years, mean 1.72 years), exacerbating factors and relieving factors.
An online, anonymised, retrospective survey was undertaken using pacemaker and ICD support sites. The Pacemaker Club online support group now claims to have over 40,500 members (https://www.pacemakerclub.com), and the Facebook groups Pacemaker Support and Living with an ICD, have 20,000 members (https://www.facebook.com/groups/952659304852414, and https://www.facebook.com/groups/icdsupport), representing approximately 4.5% of those worldwide with pacemakers and ICDs, making such members a large, but self-selected, group of the world’s total number of CIED recipients. Members of both sites voluntarily register their interest in belonging to such online support groups. Both sites are English speaking and accept members worldwide.
Appendix. The questions reported on
|
Anecdotal evidence from these support groups suggests that there is a group of implant recipients with long-term problems of pain, related to their implant, and that they are medically difficult to manage. These English-speaking support sites represent personal experiences worldwide, rather than from any specific country. Participants were given a brief summary of why the survey was taking place and the content of the survey, and were invited to take part on the understanding that their participation was entirely voluntary and their answers would be anonymised. Participants were self-selected, consenting to take part in this online survey by clicking onto a link taking them to the survey. This invitation was repeated weekly on the site’s message board by the person responsible for participant communication liaison. Responses were predominantly recorded between June 2021 and December 2021. There were 322 questionnaires (consisting of 19 questions – Appendix) returned.
Participants could opt out of answering questions, resulting in not all 19 questions being answered fully. The last question, ‘Any comments’, gave latitude for participants to express themselves as they wished. Text replies and comments were grouped into emerging themes in order to obtain grounded outcomes.
Results
Of the 322 support group respondents, the median age was 60 years (range 6–91 years): 217 (67.8%) were female, 103 male (32.2%). Two respondents opted out of their gender statement. The number of support group respondents with pacemakers was 212. The number with ICDs was 110.
The median number of years of having had their last device was one (mean 1.72 years). The frequency spread of implant duration is shown in figure 1.
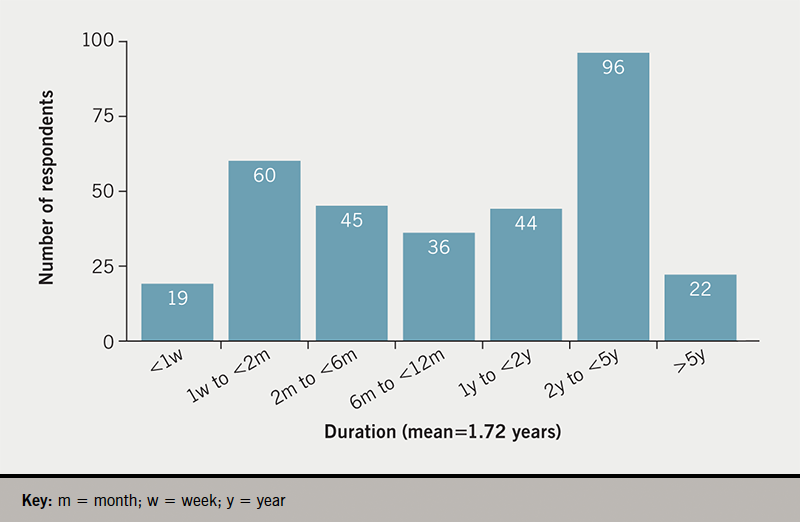
Half (50%) of the support group respondents had had their present implant for less than one year. Of respondents, 85% had received one implant, 10% had received two implants, 2% had received three implants, and 3% had received four implants. No respondent had received more than four implants. The duration of last implant was not associated with gender (mean 1.97 years for males, mean for females 1.58 years; t-test 0.79, p=0.22, not significant as p>0.05).
Of our support group respondents, 208 of the 322 participants (64.8%) reported pain for one week or more: 32 people had pain for six months or more (10.0% of respondents). Duration of pain for over two months represented 24% (95% confidence interval [CI] ±9.8%) of those responding to this question – almost one in four of those replying.
Participants were asked, ‘Using any number from 0 to 10, score your pain, where 0 is none and 10 is the worst possible pain, what number would you use to rate your pain? (slide pointer to insert value).’ The sliding pointer was to the nearest integer. Figure 2 shows the frequency of response for pain scores.
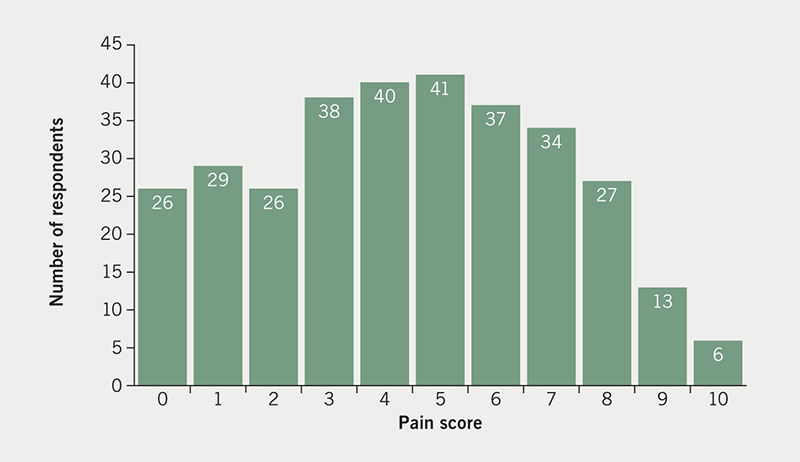
The median pain score was five. The mean pain score was found to be 4.5 (95%CI 4.2 to 4.8). The data are not normally distributed being skewed towards pain scores <6 (Kolmogorov-Smirnov test of normality D=0.093, p=0.04). The majority (62.7%) found the pain to be not of great severity (score <6, as defined by this study scale). Over a third of respondents (37.3%) found the pain to be severe (score >5), 2% describing the pain as the worst possible (i.e. score 10).
Table 1. Site of pain after implant. There were 437 sites of pain mentioned by 322 respondents
| Site of pain | Number | Percentage |
|---|---|---|
| Incision pain | 116 | 26.5% |
| Implant/pocket pain | 86 | 19.7% |
| No pain or no answer | 56 | 12.8% |
| Shoulder pain | 51 | 11.7% |
| Localised chest pain | 45 | 10.3% |
| Upper arm/arm not specified | 31 | 7.1% |
| Axillary pain | 13 | 3.0% |
| Central chest pain | 12 | 2.7% |
| Neck/jaw pain | 11 | 2.5% |
| Scapular/back pain | 8 | 1.8% |
| Lower arm pain | 7 | 1.6% |
| Elsewhere pain | 1 | 0.2% |
Pain experienced is certainly not confined to the pacemaker site (incision and implant, pocket pain), 178 other sites of pain were reported by 258 people experiencing pain (69.0% of those having pain). Responses were themed by anatomical sites (table 1). The character of the pain was said to be sharp by 33% of respondents, dull by 32% of respondents, and burning by 19%. Aching, tingling, and general soreness were also described.
Pain relief was categorised by 281 support group respondents: 122 people used paracetamol for pain relief, 56 people used ice, 45 people used non-steroidal anti-inflammatory drugs (NSAIDs), and 22 people used opiates. Other forms of pain relief used were heat, alcohol, marijuana, oil of oregano, turmeric, and gabapentin/pregabalin. No pain relief was required by 17.1% (48) of the 281 respondents. Careful positioning at night was said to be helpful. Many respondents mentioned multiple medications.
Critical comments were received from support group respondents, some of which are reproduced below:
- “It would have been helpful if they had sent me home with something for pain – they didn’t.”
- “I needed a sleep medication to sleep through the pain the first night because I was offered only Tylenol which did nothing. When I got home, ice was my friend!”
- “It was a squeezing pressure and all over body pain that required Percocet every 4 hours for 8–10 days. I cried when nurse said the doctor forgot to order it. Unbearable discomfort.”
- “The only pain relief my cardiologist approves of is Tylenol, and it does not help much.”
- “Since my doctors won’t prescribe more than 3 days of pain meds, we are forced to find alternatives. Oil of Oregano.”
- “Being sent home 5 hours after lung re-inflated without high dose pain med like morphine. It is inhumane and medically wrong.”
The complication rate reported by those support group members responding to this survey was 38.5% (124 reported individuals of 322 participants). The expected complication rate would be around 2–13% according to the pooled data from various centres.3-6,15-17
Table 2. Medical complications reported by participants (n=322)
| Medical complication | Number |
|---|---|
| Device movement from initial site | 18 |
| Lead placement problem | 16 |
| Wound infection | 13 |
| Device preventing arm movement | 12 |
| Excessive bruising or blood clot at the implant site | 11 |
| Lead fracture | 7 |
| Cosmetically unacceptable | 6 |
| Badly placed device | 4 |
| Skin break | 3 |
| Other | 34 |
| Total complications | 124 |
What is classified as a medical complication varies between centres. Medical complications reported here were themed by groups, that were mentioned by the participants (n=322) of this survey; these are shown in table 2. ‘Other’ complications (27% of the total complications) included: excessive pain during and after procedure, myocardium perforation, thoracotomy, haematoma, intravenous (IV) infusion-related swelling at the site, pneumothorax shoulder pain/frozen shoulder, psychological problems, leads badly placed, tissue glue allergy, pleural effusion, and exacerbation of hypertension.
Comparing the pain scores of those with complications and those without complications, using Mann-Whitney U-Test, no significance was found in the reported pain scores (z-score 1.15, p=0.25; the scores were approximately normally distributed). The mean pain score for the complication group was 4.26, and for the non-complication group 4.64.
Of those (n=124) reporting complications, one person had pain of 6–12 months’ duration, and two had pain of 1–2 years’ duration; 56 reported pain from one week to two months’ duration. Long-term pain does not seem to be associated with complication rates in this study.
‘Major complications’ (pre-defined and adjudicated by a committee) have been reported to be higher with implantable ICDs compared with pacemaker replacements.18 This was not found to be the case in this retrospective survey’s reported complication rate in these groups (Chi-square statistic 0.66, p=0.42, not significant).
Discussion
This worldwide, English-speaking, survey of online support group members showed a relatively high proportion (38.5%) of the 322 support group respondents having complications from their implant. This depends on how you define ‘complications’. This is especially a problem when a survey is patient-orientated (as this survey), and includes complications such as cosmetically unacceptable, or badly placed device (these two complications accounting for 10% of reported complications), compared with ‘medical complications’ reported in medical literature.15-17 Pain is not necessarily seen as a complication of a CIED procedure.3-6 The responses obtained are from a group of CIED recipients finding the need for self-support. We should, as healthcare professionals, ask why this form of support is needed?
The advice that, ‘minor wound pain is expected after device implantation, almost always controlled with simple analgesia’,10 would seem to be inappropriate, as found by this study when the duration of pain for over two months, represented 24% (95%CI ±9.8%) of those responding to this question – almost one in four of those replying.
Furthermore, the pain severity was for over a third of respondents (37.3%) found to be severe (score >5), 2% describing the pain as the worst possible (score 10). This adds support to previous findings by Lee et al.,11 that peri-operative pain management in CIED procedures warrants meticulous attention. Pain from pacemaker and ICD implant surgery remains a problem both in terms of severity and longevity.
Our respondents are a self-selected group seeking support from each other, mostly outside of medical professionals. Personal experience may not transfer into generality. However, comments received related to a number of disturbing physician findings:
- “I had to beg the doctor for pain medication after the procedure.”
- “My doctor keeps saying it should only be mild discomfort, which makes me feel like they are dismissing my pain/experience. Not everyone has the same experience, pain level, etc. My pain is real.”
- “The doctors were no help. Once they put it in, they were done.”
- “Some members of my medical team belittled my pain concerns and refused to give me pain meds. It was an awful experience.”
- “Most doctors underestimate the amount of pain post-procedure.”
- “I wish doctors would make it clear about getting a pacemaker and the complications that come with it.”
There would appear to be a need for better information given to patients, as found by this survey, and summed up by this comment:
- “I found the literature about pacemakers misleading, as they said most people are fine after a day and back to work in a week. My boss expected this too. But I was uncomfortable, tired and emotional, and felt a failure for not being back to normal in a week as suggested. More realistic info needed.”
This worldwide survey, of those English-speaking CIED patients seeking a self-help group, highlights a need for better medical communication to CIED patients. Advice to patients, and the literature they receive at the time of the procedure, needs to change. It should now be seen that pain from pacemaker and ICD implant surgery remains a problem for some patients, both in terms of severity and longevity. This should be clear to both patient and physician. Severe pain and enduring pain needs to be recorded as a complication on follow-up of CIED patients. This should then act as a stimulus for further studies to better explore why such pain occurs and what is the best management for such pain.
Key messages
- Pain from pacemaker and implantable cardioverter-defibrillator (ICD) implant surgery remains a problem both in terms of severity and longevity for some patients
- In the assessment of the patient experience of pain following implantation of cardiac pacemakers and ICDs, there was found to be a need for better pain management and realistic communication with patients belonging to self-help groups
- Further research is needed into the problems of chronic and severe pain associated with these procedures
Conflicts of interest
None declared.
Funding
None.
Study approval
Ethical approval for this study was obtained from the moderator of the Pacemaker Club (https://www.pacemakerclub.com).
Acknowledgements
With thanks to The Pacemaker Club (https://www.pacemakerclub.com), and Pacemaker Support and Living with an ICD Facebook groups (https://www.facebook.com/groups/952659304852414, and https://www.facebook.com/groups/icdsupport). Also to Therese Paolini – participant communication liaison.
References
1. Glikson M, Nielsen JC, Kronborg MB et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427–520. https://doi.org/10.1093/eurheartj/ehab364
2. Stewart C. Global number of pacemakers in 2016 and 2023. Statista.com, 20 April 2022. Available at: https://www.statista.com/statistics/800794/pacemakers-market-volume-in-units-worldwide/
3. Shakya S, Matsui H, Fushimi K, Yasunaga H. In-hospital complications after implantation of cardiac implantable electronic devices: analysis of a national inpatient database in Japan. J Cardiol 2017;70:405–10. https://doi.org/10.1016/j.jjcc.2017.02.013
4. Khanal J, Poudyal RR, Devkota S, Thapa S, Dhungana RR. Clinical profile and early complications after single and dual chamber permanent pacemaker implantation at Manmohan Cardiothoracic Vascular and Transplant Centre, Kathmandu, Nepal. J Nepal Health Res Counc 2015;13:138–43. Available from: https://www.jnhrc.com.np/index.php/jnhrc/article/view/638/497
5. Møller M, Arnsbo P, Asklund M et al. Quality assessment of pacemaker implantations in Denmark. Europace 2002;4:107–12. https://doi.org/10.1053/eupc.2002.0234
6. Gadler F, Valzania C, Linde C. Current use of implantable electrical devices in Sweden: data from the Swedish pacemaker and implantable cardioverter-defibrillator registry. Europace 2015;17:69–77. https://doi.org/10.1093/europace/euu233
7. Pavia S, Wilkoff B. The management of surgical complications of pacemaker and implantable cardioverter-defibrillators. Curr Opin Cardiol 2001;16:66–71. https://doi.org/10.1097/00001573-200101000-00010
8. Ludwig S, Theis C, Wolff C, Nicolle E, Witthohn A, Götte A. Complications and associated healthcare costs of transvenous cardiac pacemakers in Germany. J Comp Eff Res 2019;8:589–97. https://doi.org/10.2217/cer-2018-0114
9. Kiviniemi MS, Pirnes MA, Eränen HJ, Kettunen RV, Hartikainen JE. Complications related to permanent pacemaker therapy. Pacing Clin Electrophysiol 1999;22:711–20. https://doi.org/10.1111/j.1540-8159.1999.tb00534.x
10. Schiariti M, Cacciola M, Puddu PE. Chapter 15. Complications of pacemaker implantation. In: Das MM (ed.). Modern Pacemakers – Present and Future. London: IntechOpen, 2011; pp. 271. https://doi.org/10.5772/556
11. Lee JZ, Pasha AK, Glasgow AE et al. Postoperative opioid prescription patterns and new opioid refills following cardiac implantable electronic device procedures. Heart Rhythm 2019;16:1841–8. https://doi.org/10.1016/j.hrthm.2019.08.011
12. Lee JZ, Glasgow AE, Habermann EB et al. Liposomal bupivacaine during subcutaneous implantable cardioverter defibrillator implantation for pain management. Pacing Clin Electrophysiol 2021;44:513–18. https://doi.org/10.1111/pace.14175
13. Yang JK, Char DS, Motonaga KS et al. Pectoral nerve blocks decrease postoperative pain and opioid use after pacemaker or implantable cardioverter-defibrillator placement in children. Heart Rhythm 2020;17:1346–53. https://doi.org/10.1016/j.hrthm.2020.03.009
14. NHS Inform. Pacemaker implantation. 3. Recovering from pacemaker implantation. Will I be in pain after the procedure? November 2021. Available at: https://www.nhsinform.scot/tests-and-treatments/surgical-procedures/pacemaker-implantation#recovering-from-pacemaker-implantation
15. Kotsakou M, Kioumis I, Lazaridis G et al. Pacemaker insertion. Ann Transl Med 2015;3:42. https://doi.org/10.3978/j.issn.2305-5839.2015.02.06
16. van Rees JB, de Bie MK, Thijssen J et al. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: a systematic review of randomized clinical trials. J Am Coll Cardiol 2011;58:995–1000. https://doi.org/10.1016/j.jacc.2011.06.007
17. Pakarinen S, Oikarinen L, Toivonen L. Short-term implantation-related complications of cardiac rhythm management device therapy: a retrospective single-centre 1-year survey. Europace 2010;12:103–08. https://doi.org/10.1093/europace/eup361
18. Poole JE, Gleva MJ, Mela T, Chung MK. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures. Circulation 2010;122:1553–61. https://doi.org/10.1161/CIRCULATIONAHA.110.976076
