Aortic dissection is a life-threatening condition that is often under-recognised. In the first in a series of articles about the condition, the epidemiology, pathology, classification and clinical presentation of aortic dissection are discussed.
Epidemiology
|
King George II “On 25 October 1760 George II, then 76, rose at his normal hour of 6 AM, called as usual for his chocolate, and repaired to the closet-stool. The German valet de chambre heard a noise, memorably described as ‘louder than the royal wind’, and then a groan; he ran in and found the King lying on the floor, having cut his face in falling. Mr Andrews, surgeon of the household, was called and bled his Majesty but in vain, as no sign of life was observed from the time of his fall. At necropsy the next day Dr Nicholls, physician to his late Majesty, found the pericardium distended with a pint of coagulated blood, probably from an orifice in the right ventricle, and a transverse fissure on the inner side of the ascending aorta 3.75 cm long, through which blood had recently passed in its external coat to form a raised ecchymosis, this appearance being interpreted as an incipient aneurysm of the aorta.” Taken from King George II Biography – Facts, Childhood, Family Life & Achievements (thefamouspeople.com) |
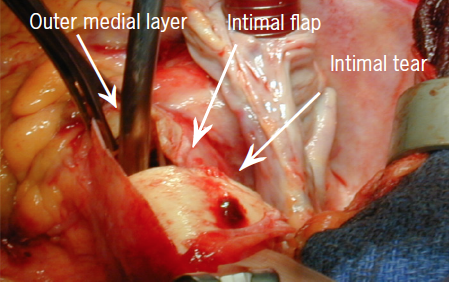
Aortic dissection was described historically as far back as the time of King George II (box),1 and is now understood better in modern times. It is rare in medicine that a new disease is discovered, more that over time we come to understand its pathology and natural history better. An aortic dissection is a catastrophic event inside the biggest blood vessel inside the body (figure 1). A tear on the innermost lining of the vessel, which creates false channels, enlargement of the size of the vessel and rupture, as well as lack of blood to some of the most vital organs inside the body, it gets little ‘press’ compared with other diseases such as cancer and heart disease. The incidence of aortic dissection is rarely published. Due to how diseases and deaths are recorded we have information on what happens to patients who are treated via hospital admission, but those who die in the community without a post-mortem often go unrecorded. As many of half of all who suffer an aortic dissection, die before making it to a hospital.
For this article, to establish the recorded incidence of aortic dissection in the UK, the lead author reviewed Office for National Statistics (ONS) data for 1998–2019.2 ONS data use the International Classification of Diseases (ICD) to classify cause of death. This means, the number of death registrations where the underlying cause was aortic aneurysm and dissection, listed by sex and five-year age group in England and Wales could be reviewed. The ONS data for the population of the UK in mid-2020 was 67 million, of which 59,597,300 people resided in England and Wales. There were 4,106 deaths registered as aortic aneurysm and dissection (0.007%), which is equivalent to seven per 100,000 population. The average age was over 60 years and the majority were male. We, therefore, can infer that in a city or region of the UK with a population of 2 million people, roughly 140 people per year will die from this disease. Many more will have been diagnosed and survived.
Throughout the world, other studies of large registries of patients suffering aortic dissection, such as the International Registry of Aortic Dissection (IRAD) paper of 1998, study, not incidence, but symptoms, initial investigation, management, and outcomes.3 This means any patient admitted was reviewed and included in this prospective study. As of 31 December 1998, 464 patients had been enrolled. Two thirds of those patients were male. The mean age of all patients was 63.1 years (95% confidence interval [CI] 61.8 to 64.4 years). Type A dissection was identified in 62.3% of patients. Patients with type B dissection were, on average, older (p<0.001). A history of cardiac surgery was present in 83 patients (17.9%). Iatrogenic dissection was reported in 20 patients (4.3%). Sixty per cent of patients initially presented to an outside hospital and were referred to IRAD centres for continued management. A history of hypertension was elicited in 72.1% of all patients. Marfan syndrome was present in 4.9% of all patients (mean age 36 years; range 13–52 years). Surgical management occurred in 72% and 28% were not offered suitable intervention due to comorbidity, intramural haematoma or death prior to intervention.
From this we can ascertain that dissection is generally a disease of older age, mostly caused by hypertension, and is more common in men.
To really understand epidemiology, published small population-based studies are limited, but are combined in table 1.4–15 Large national registries, such as those in Japan or Spain, provide substantial data on the management of patients with acute aortic dissection (AAD), they can only display a partial view of the whole natural history, because of the incomplete inclusion of patients deceased prior to medical assistance and diagnosis. Yet, population-based studies including death cases provide a better estimation of the actual epidemiology of AAD.4–15
Table 1. The incidence and outcomes of acute aortic dissection (AAD) in different population-based studies4–15
| Study | Melvinsdottir et al. | Howard et al. | Smedberg et al. | Yeh et al. | Lee et al. | Dinh et al. | Yamaguchi et al. |
|---|---|---|---|---|---|---|---|
| Study period | 1992–2013 | 2002–2012 | 2002–2016 | 2005–2012 | 2005–2016 | 2017–2018 | 2016–2018 |
| Population | Iceland | Oxfordshire, UK | Sweden | Taiwan | Korea | NSW, Australia | Miyazaki, Japan |
| Demographic | Nationwide | Regional | Nationwide | Nationwide | Nationwide | Regional | Regional |
| Number with AAD | 153 | 52 | 8,057 | 9,092 | 18,565 | 273 | 79 |
| Mean age, years | 66.9 | 72 | 68 | 64.4 | 67 | – | 76 |
| Incidence per 105 inhabitants | 2.53 | 6 | 7.2 | 5.6 | 3.76 | 3.47 | 17.6 |
| Deaths included | Yes | Yes | Yes | No | No | No | Yes |
| Emergency surgery, % | 43.7 | 36.5 | 32 | 38.3 | – | 51 | 30 |
| 30-day mortality | 45.2 | 55.8 | 23 | 17.7 | 10.8 | 35.5 | 74.5 |
In the papers referenced in table 1, Yamaguchi and colleagues report an AAD incidence as high as 17.6 per 100,000 inhabitants in a population-based study conducted from 2016 to 2018 in the Miyazaki prefecture in Japan. Although the study period was relatively short (three years), and the geographical area relatively limited, the age-adjusted design had the potential to provide representative data about AAD incidence and mortality in that country. In contrast to previous studies, the authors used post-mortem non-injected computed tomography (CT) to clarify the causes of deaths, as a less complex alternative to full autopsy.
Interestingly, almost half of patients included (46.8%) were dead at hospital arrival. The autopsy rate has continuously decreased, and varies widely between countries and hospitals. Two previous Japanese studies have validated the use of non-injected CT scan in such a setting. While non-injected CT scan may miss limited aortic lesions (e.g. penetrating ulcer), it is implausible that acute aortic syndromes with limited lesions lead to patient death without extensive dissection and/or rupture.
Such a high AAD incidence, reported by Yamaguchi et al., which represents almost twice the value in previous reports, could be mostly explained by an older mean age of patients (76 years) in the country with the highest life-expectancy, and the fact that almost all patients (96.5%) with cardiopulmonary arrest at arrival underwent CT investigation. Regarding outcome, Yamaguchi et al. reported an overall AAD 30-day mortality rate as high as 57%: only one patient out of four survived in case of type A AAD while one out of four died in the presence of type B AAD. Overall, the age-adjusted 30-day mortality of AAD per 100,000 inhabitants was 9.9, a rate much higher than that reported in previous studies, that could again be explained by a better capture of pre-hospital deaths. For instance, 60% of type A AAD were in cardiac arrest at hospital arrival.
Although the interval from the symptom onset to arrival at hospital was short, the outcome of AAD was poor. The high incidence and mortality of such a life-threatening condition with limited management alternatives, underline the importance of prevention. Long history of uncontrolled or undiagnosed hypertension and the presence of aortic aneurysm represent the main risk factors associated with AAD, followed by bicuspid aortic valve and genetic connective tissue disorders.
Pathology and natural history
AAD is one of the most devastating cardiovascular diseases, with a high risk of death. The aorta is made up of four main layers, and some describe it as similar to an onion, in which layers can be peeled away. This is useful when we think of the pathophysiology of aortic dissection in which the innermost layer, the intima, is damaged and then stripped away in a catastrophic event (dissection), making a single tube of aorta into a tube with two separate lumens. The typical presentation is characterised by acute onset of severe pain. However, clinical manifestations are diverse, and what were previously considered to be classic symptoms and signs are often absent. Therefore, a high clinical index of suspicion is necessary. With so many other causes of chest pain more prevalent in the mind of clinicians, it is so important that we ‘Think Aorta’.
Diagrammatic representation of aortic dissection is seen in figure 2. A tear in the aortic intima leads to redirection of the blood flow into the media, creating two tubes within one. The inner true lumen (TL) and an outer false lumen (FL), separated by the dissection flap. The tear is caused by cystic medial necrosis or by progression of an intramural haematoma (IMH) or a penetrating aortic ulcer (PAU). The intimal tear usually occurs in the ascending aorta, 2 to 3 cm above the aortic valve, but can also occur within the aortic root, the arch or the distal thoracic aorta (DTA). The dissection may then extend antegradely for a variable distance, and may compromise the perfusion of the organs supplied by the side branches of the aorta, although both lumens can communicate distally through the presence of fenestrations. It can also extend retrogradely, towards the aortic valve and the coronary ostia, causing aortic regurgitation and myocardial ischaemia, respectively. In short, the complications of the dissection, tamponade, heart attack, stroke, abdominal organ and leg ischaemia are the complications that cause the death of the patient.

Classification
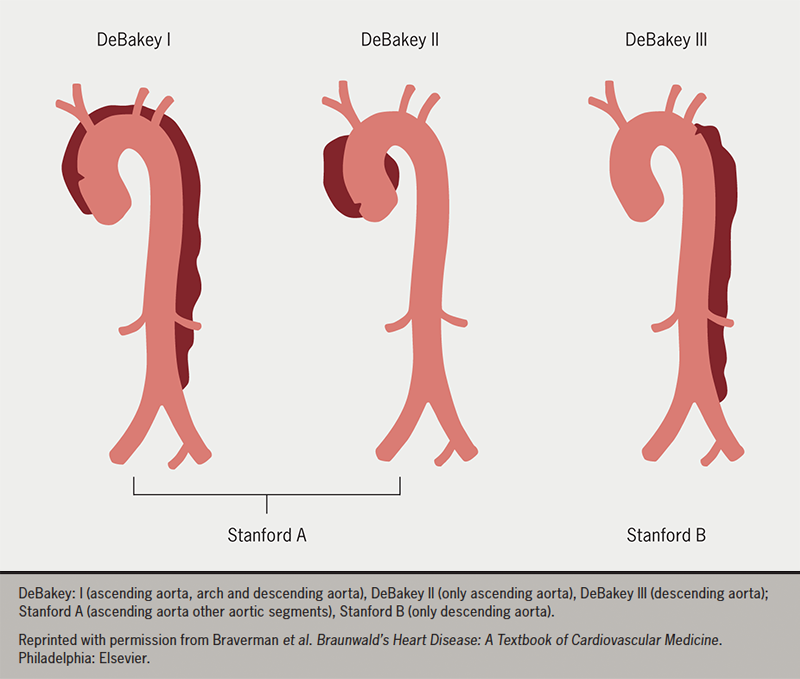
Classification systems for aortic dissection (AD) provide important guides to clinical decision-making. The oldest systems were anatomic or temporal (figure 3). The DeBakey classification system of Types I, II, IIIa, and IIIb were published in 1965, followed by the simpler Stanford classification (Type A and B) in 1970, based on the involvement of the ascending aorta. Dissection was classified as acute (<14 days), subacute (15 to 92 days), or chronic (>90 days), depending on the timing of onset of symptoms.
Clinical presentation
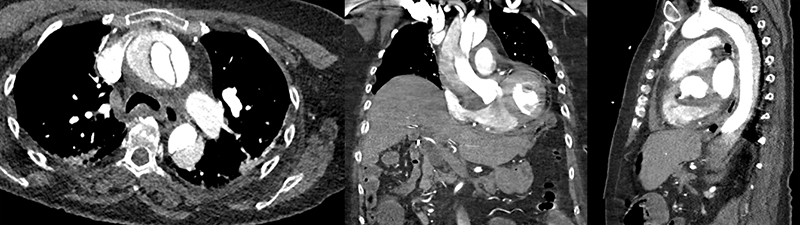
High index of suspicion is usually required for the diagnosis, as the presentation might be initially similar to more frequent conditions, such as a heart attack (acute coronary syndrome) or pulmonary embolism. The most frequent symptom is severe chest and/or back pain, described as an abrupt, tearing or ripping sensation; it could migrate from its point of origin, following the dissection path as it extends through the aorta. Abdominal pain can also be a presenting symptom, but less frequently. If the dissection affects the competency of the aortic valve, the patient will present with signs and symptoms of acute heart failure, due to the poorly tolerated acute overload of a nondilated left ventricle. The clinical presentation of acute aortic syndrome (AAS), including AD, ranges from incidental detection to acute onset with chest pain, and symptoms of progressive perfusion deficits and end-organ ischaemia. The classical presentation is described as acute, severe, tearing chest pain (TAAD), or back pain (TBAD), but this can also be described as a sharp or stabbing pain. A high index of suspicion and early imaging is critical for diagnosis (figure 4), especially because TAAD is associated with an hourly mortality rate of 1%.
The dissection can rupture into the pericardium and progress into cardiac tamponade, presenting with hypotension or even sudden death. Depending on the progression of the dissection compromising the blood supply to different aortic branches, either by dissection of the branch itself or by mechanical compression induced by the FL, the following complications might occur:
- Stroke related to malperfusion to the brain, due to involvement of the supra-aortic trunks.
- Mesenteric ischaemia, which is usually insidious and might be diagnosed too late when the bowel is already not viable. High suspicion and identification via increasing lactate levels are paramount for its diagnosis.
- Acute renal failure, due to hypoperfusion or prolonged hypotension, is identifiable by monitoring of urine output and serial creatinine.
- Lower limb ischaemia or spinal cord ischaemia, presenting with acute paraplegia.
Risk factors
AAD and broader AAS are linked to conditions with increased wall stress (e.g. uncontrolled hypertension, phaeochromocytoma, cocaine or other stimulant use, weightlifting, trauma with deceleration or torsional injury, and aortic coarctation) and conditions with connective tissue abnormalities (e.g. Marfan, Loeys–Dietz, Ehlers–Danlos type 4, Turner syndrome, familial thoracic aortic aneurysm in dissection syndrome, bicuspid aortic valve associated with NOTCH, and other aortopathies).
Screening and prevention
Screening for congenital aortopathies is based on the identification of specific genotypes known to predispose patients to dissection. Identification of a specific gene influences the size at which repair is considered (4.5 cm for Marfan, 4.2 cm for Loeys-Dietz, 4.5 cm for Moyamoya, and 5 cm for Ehlers–Danlos), as well as the type of repair.
Summary
The incidence of aortic dissection in the UK is 7–10 per 100,000 population, meaning that in an area with a population of two million people, over 140 will suffer from this disease annually and half will die before making it to hospital. A life-threatening and time-dependent condition, clinicians must ‘Think Aorta’ to diagnose it, as the most common symptom is chest pain, often attributable to other heart conditions. It is commonly seen in those in their seventh decade of life, in males, and appears in younger patients with congenital aortopathies, and needs a high index of suspicion. The pain is the tearing of the inside of the innermost layer of the aorta, the aortic intima, and has a characteristic appearance on CT scan (like a picture of a tennis ball), which is the easiest method to diagnose it.
Key messages
- Aortic dissection occurs at a rate of roughly 7 per 100,000 population and only half of those who present with it make it to hospital
- Aortic dissection is a life-threatening condition recognised by a strong history of severe chest or back pain
- The computed tomography (CT) findings for diagnosis are a classic image of two lumens created in the aorta, which normally only has one
Conflicts of interest
None declared.
Funding
None.
Editors’ note
See also the editorial from this issue, which can be found at https://doi.org/10.5837/bjc.2023.008.
References
1. Trench CC. George II. London: Allen Lane, 1973;pp. 298.
2. Office for National Statistics. Number of death registrations where the underlying cause was aortic aneurysm and dissection by sex and five year age group, England and Wales: 1998 to 2019. London: ONS, 28 October 2020. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/12426numberofdeathregistrationswheretheunderlyingcausewasaorticaneurysmanddissectionbysexandfiveyearagegroupenglandandwales1998to2019
3. Hagan PG, Nienaber CA, Isselbacher EM et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897–903. https://doi.org/10.1001/jama.283.7.897
4. Pacini D, Di Marco L, Fortuna D et al. Acute aortic dissection: epidemiology and outcomes. Int J Cardiol 2013;167:2806–12. https://doi.org/10.1016/j.ijcard.2012.07.008
5. Melvinsdottir IH, Lund SH, Agnarsson BA, Sigvaldason K, Gudbjartsson T, Geirsson A. The incidence and mortality of acute thoracic aortic dissection: results from a whole nation study. Eur J Cardiothorac Surg 2016;50:1111–17. https://doi.org/10.1093/ejcts/ezw235
6. Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM; Oxford Vascular Study. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031–7. https://doi.org/10.1161/CIRCULATIONAHA.112.000483
7. Smedberg C, Steuer J, Leander K, Hultgren R. Sex differences and temporal trends in aortic dissection: a population-based study of incidence, treatment strategies, and outcome in Swedish patients during 15 years. Eur Heart J 2020;41:2430–8. https://doi.org/10.1093/eurheartj/ehaa446
8. Yeh TY, Chen CY, Huang JW, Chiu CC, Lai WT, Huang YB. Epidemiology and medication utilization pattern of aortic dissection in Taiwan: a population-based study. Medicine (Baltimore) 2015;94:e1522. https://doi.org/10.1097/01.md.0000481359.77220.0b
9. Lee JH, Cho Y, Cho YH et al. Incidence and mortality rates of thoracic aortic dissection in Korea – inferred from the nationwide health insurance claims. J Korean Med Sci 2020;35:e360. https://doi.org/10.3346/jkms.2020.35.e360
10. Dinh MM, Bein KJ, Delaney J, Berendsen Russell S, Royle T. Incidence and outcomes of aortic dissection for emergency departments in New South Wales, Australia 2017–2018: a data linkage study. Emerg Med Australas 2020;32:599–603. https://doi.org/10.1111/1742-6723.13472
11. Yamaguchi T, Nakai M, Sumita Y et al. Current status of the management and outcomes of acute aortic dissection in Japan: analyses of nationwide Japanese registry of all cardiac and vascular diseases – diagnostic procedure combination data. Eur Heart J Acute Cardiovasc Care 2020;9(suppl 3):S21–S31. https://doi.org/10.1177/2048872619872847
12. Evangelista A, Rabasa JM, Mosquera VX et al. Diagnosis, management and mortality in acute aortic syndrome: results of the Spanish Registry of Acute Aortic Syndrome (RESA-II). Eur Heart J Acute Cardiovasc Care 2018;7:602–08. https://doi.org/10.1177/2048872616682343
13. Yamaguchi T, Nakai M, Yano T et al. Population-based incidence and outcomes of acute aortic dissection in Japan. Eur Heart J Acute Cardiovasc Care 2021;10:701–09. https://doi.org/10.1093/ehjacc/zuab031
14. Acosta S, Gottsäter A. Stable population-based incidence of acute type A and B aortic dissection. Scand Cardiovasc J 2019;53:274–9. https://doi.org/10.1080/14017431.2019.1642509
15. Tanaka Y, Sakata K, Sakurai Y et al. Prevalence of type A acute aortic dissection in patients with out-of-hospital cardiopulmonary arrest. Am J Cardiol 2016;117:1826–30. https://doi.org/10.1016/j.amjcard.2016.03.015
