We sought to remedy the limited guidance that is available to support the resuscitation of patients with the Impella Cardiac Power (CP) and 5.0 devices during episodes of cardiac arrest or life-threatening events that can result in haemodynamic decompensation.
In a specialist tertiary referral centre we developed, by iteration, a novel resuscitation algorithm for Impella emergencies, which we validated through simulation and assessment by our multi-disciplinary team. A mechanical life support course was established to provide theoretical and practical education, combined with simulation to consolidate knowledge and confidence in algorithm use. We assessed these measures using confidence scoring, a key performance indicator (the time taken to resolve a suction event) and a multiple-choice question (MCQ) examination.
Following this intervention, median confidence score increased from 2 (interquartile range [IQR] 2 to 3) to 4 (IQR 4 to 4) out of a maximum of 5 (n=53, p<0.0001). Theoretical knowledge of the Impella, as assessed by median MCQ score, increased from 12 (IQR 10 to 13) to 13 (12 to 14) out of a maximum of 17 (p<0.0001).
The use of a bespoke Impella resuscitation algorithm reduced the mean time taken to identify and resolve a suction event by 53 seconds (95% confidence interval 36 to 99, p=0.0003).
In conclusion, we present an evidence-based resuscitation algorithm that provides both technical and medical guidance to clinicians responding to life-threatening events in Impella recipients.
Introduction
The Johnson and Johnson Inc. (formerly Abiomed Inc.) Impella left ventricular assist devices (LVADs) are catheter-based, intravascular, transaortic micro-axial flow pumps that supplement native ventricular function while providing ventricular offloading. There are a range of left ventricular Impella devices of increasing size from Impella 2.5, Impella Cardiac Power (CP), Impella 5.0 and Impella 5.5. The Impella 2.5 and CP are inserted via a percutaneous femoral access route, and provide up to 3.5 L/min blood flow, whereas the Impella 5.0 is deployed surgically via a branch graft to the subclavian artery and provides up to 5 L/min. Impella support is increasingly applied both during primary coronary intervention,1 and for medium-term use during cardiogenic shock.2-4 The Impella 5.5 provides up to 5.5 L/min support, and a right-sided device is also available. Within the Impella CP catheter, a pressure transducer allows the proximal aortic pressure to be continuously monitored. This is referred to as the placement signal. In contrast, for the Impella 5.0 the placement signal represents the pressure gradient profile across the aortic valve. This reaches a maximal value in ventricular diastole and a minimum in ventricular systole. For all Impella LVADs, systemic perfusion is normally supplemented by residual left ventricular output. The P-level corresponds to the level of blood flow through the device, and ranges from no flow at P0 to maximum flow at P9. The purge system supplies constant pressurised fluid (typically 5% dextrose containing heparin) through the device, maintaining high pH, anticoagulation and fluid-blood barrier to protect the pump.
Catheter laboratory staff in centres that deploy the Impella have become experienced in its preparation and insertion using the rapid imaging methods of fluoroscopy and echocardiography. However, for the therapy to be effective in both the catheter laboratory and in the intensive-care setting (if prolonged support is required), complications must be recognised early and remedial actions implemented without delay.
Impella therapy-associated complications, in our experience, fall into five broad categories: misplacement of the catheter during insertion resulting in impaired blood flow; unintentional displacement of the catheter, particularly as a result of skin-care and patient transfer manoeuvres; impaired flow through the Impella as a result of inflow occlusion (suction) or pump thrombosis; excessive blood trauma; and compromise to the integrity of the catheter and/or the purge system.
The manufacturer provides comprehensive online troubleshooting guides for the Impella systems,5 including remedial instructions for common issues. With the advent of the Impella connect platform,6 console alarms can be accessed remotely and alert physicians in a timely manner. However, a quick reference tool that embodies the principles of both resuscitation using the airway, breathing, circulation (ABC) approach for deteriorating patients or those in cardiac arrest, and device troubleshooting, is not yet available.
We have previously described the development of emergency resuscitation algorithms for patients with implantable LVADs for use in the pre-hospital and inpatient settings.7,8 We applied a similar strategy in the development of an Impella resuscitation algorithm and training structure, as described.
Method
This initiative was conducted between November 2021 and August 2022 at Harefield Hospital, part of Guy’s and St. Thomas’ NHS Foundation Trust, a regional transplant and mechanical circulatory support (MCS) centre with an active primary angioplasty service. We deploy the Impella CP for peri-procedural use in the catheter laboratory, and the Impella CP and 5.0 for cardiogenic shock, as a bridge to transplantation.9 The Impella CP may also be deployed for left ventricular decompression during veno-arterial extracorporeal membrane oxygenation (VA-ECMO).4 The guidance described only pertains to the use of isolated Impella LVADs, i.e. it is not applicable when the Impella is deployed in combination with other MCS devices.
Algorithm design
We convened a working group consisting of key medical, nursing and allied healthcare professional stakeholders in cardiology, intensive care, perfusion, cardiac surgery and resuscitation. Through a process of simulation and testing, the algorithm was iterated until it was deemed to be safe and clinically efficacious (figure 1). The algorithm was refined following presentation at hospital governance meetings, was approved for internal use in February 2022, and has since been attached to all cardiac arrest trollies in clinical areas where the Impella is used.
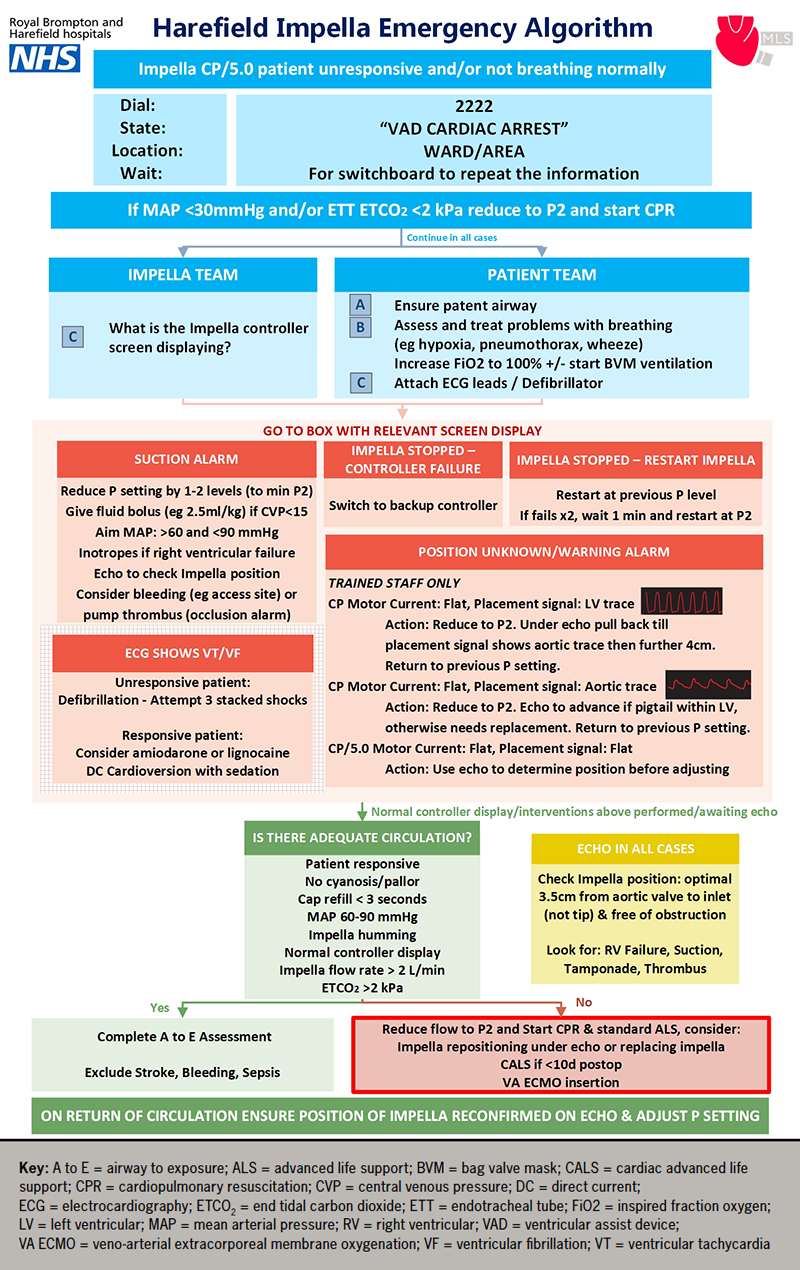
Algorithm features
The deployment of the Impella resuscitation algorithm is intended to be coordinated by a team leader whose first task should be to divide available staff into ‘Impella’ and ‘patient’ teams to allow concurrent management of MCS and medical issues, respectively.
Initial response
The algorithm starts with the identification of an Impella CP or 5.0 recipient who is unresponsive and/or not breathing normally. This leads to the activation of a ‘Ventricular Assist Device (VAD) Cardiac Arrest’ call, which alerts, not only the cardiac arrest team, but also our specialist ventricular assist device nursing team members who have a specialised knowledge of the Impella systems. We also encourage the use of the emergency call if there is any unexplained clinical deterioration.
The next step is to determine whether cardiopulmonary resuscitation (CPR) should be initiated according to a mean arterial pressure (MAP) <30 mmHg and/or an endotracheal tube end-tidal carbon dioxide (ETCO2) of <2 kPa (if recorded). The MAP threshold was determined through expert consensus and is included because all Impella recipients routinely undergo invasive arterial line monitoring. Not all patients are intubated and, thus, the ETCO2 may not always be monitored. However, when an endotracheal ETCO2 is present, a threshold of <2 kPa is recommended for the initiation of CPR in MCS.10 Prior to the initiation of CPR, it is important to reduce the Impella speed/power setting to P2 to avoid damage to the aortic valve, which could occur as a result of CPR-induced catheter displacement. Aside from aortic valve entrainment, CPR is not without risk in these patients, with chest compression potentially leading to device dislodgement,11 or pericardial perforation.12 However, pragmatically, the priority remains providing cerebral blood flow in this situation and, thus, CPR is advised until inadequate circulation is addressed.
Impella and patient teams
Irrespective of whether CPR has been initiated, the patient team should follow a standard ABC approach to secure the airway, manage any respiratory issues and attach electrocardiography monitoring and defibrillator electrodes. The Impella team should proceed to the device troubleshooting section and respond appropriately to the alarm displayed on the Impella console as detailed below.
Suction alarm: This is typically caused by inadequate blood volume within the left ventricle relative to the Impella speed (P-level) setting or by mechanical obstruction of the Impella catheter inflow. This should be managed by a reduction in the P-level by 1–2 levels and targeted fluid bolus delivery at 2.5 ml/kg. An alternative strategy would be to reduce the power setting to P-level 2 and then increase it in increments. However, selection of P-level values of less than 2 are not recommended as these can cause retrograde blood flow within the Impella (from aorta to left ventricle). Echocardiography is essential to confirm appropriate catheter position, as suction events can be caused by obstruction of the Impella catheter inflow by the ventricular septal wall/mitral valve apparatus. If malpositioned, the catheter should be repositioned by a suitably trained team member. This entails unlocking the extracorporeal portion of the catheter to allow repositioning. It is essential that the catheter is re-secured once it has been correctly repositioned. Alternative causes of suction include bleeding from the catheter access site resulting in hypovolaemia, or pump thrombosis, which is frequently associated with an occlusion alarm. Having successfully repositioned the Impella catheter, it is vital that the P-level is returned to its baseline value in order to restore full circulatory support.
Having verified correct Impella catheter placement and restored the Impella speed (P-level) to the baseline level, it is also important to maintain the MAP within the 60–90 mmHg range for two reasons: it reduces the pressure gradient across the Impella, thereby, maximising its blood flow; and it reduces left ventricular afterload and, thereby, maximises stroke volume. Inotropes should be considered if the predominant problem is right ventricular failure resulting in inadequate left ventricular filling.
Impella stopped – controller failure: When this alarm is displayed, the Impella team should follow the sequential instructions for console replacement.
Impella stopped – restart Impella: The team should restart the Impella at the previous P-level if the device has stopped. If this fails, two re-attempts should be made. If these fail, the team should wait for one minute before restarting at P-level 2, as per the manufacturer’s recommendation.5 The resumption of device operation can be checked by chest auscultation.
Position unknown/warning alarm: The Impella CP catheter is particularly vulnerable to displacement. The management of this issue requires the presence of appropriately trained staff who should be familiar with both echocardiography and the catheter repositioning process. For the Impella CP, if a left ventricular placement signal is present and the motor current trace is non-pulsatile, the speed should be adjusted to P-level 2 to avoid damaging the aortic valve, and the catheter should be withdrawn under echocardiography until an aortic pressure waveform is first observed, followed by further withdrawal, typically of approximately 4 cm. If an Impella CP displays an aortic placement signal and a non-pulsatile or almost non-pulsatile motor current waveform, the catheter should be advanced under echocardiography, but only if the pigtail remains on the ventricular aspect of the aortic valve. Otherwise, the catheter cannot be repositioned safely and a replacement Impella or alternative MCS device should be deployed.
For both the Impella CP and 5.0, a non-pulsatile placement signal in combination with a non-pulsatile motor current can be indicative of cardiac akinesia. For the Impella 5.0, non-pulsatile placement and motor current waveforms are suggestive of catheter misplacement, necessitating urgent repositioning. The Impella 5.0 placement signal can be counter-intuitive to clinicians, and so interpretation requires expert review and echocardiography to determine whether the catheter is malpositioned. The surgical placement of Impella 5.0 via a subclavian vessel side graft normally offers sustained positional stability making catheter displacement extremely unlikely, even during patient transfer and rehabilitation manoeuvres.
ECG shows VT/VF: A rapid fluctuation in placement and motor signals waveforms may be indicative of life-threatening ventricular arrhythmias, i.e. ventricular tachycardia/fibrillation (VT/VF), which can be confirmed by electrocardiographic (ECG) monitoring. If the patient is unresponsive, they should be defibrillated using three escalating stacked shocks. If the patient remains responsive, which can occur in LVAD recipients with VF/VT,6 treatment options include medical therapy (a trial of amiodarone or lignocaine) or direct current cardioversion after appropriate assessment and sedation.
Is there adequate circulation?
After initial troubleshooting there should be a re-assessment of circulatory adequacy. We have defined the following criteria to be representative of a normal circulation in an Impella recipient: the patient should be responsive; without cyanosis; with normal capillary refill (<3 seconds); MAP between 60 and 90 mmHg; a humming sound should be present on chest auscultation; there should be a normal console display with an Impella flow rate over 2 L/min; and an ETCO2 >2 kPa. If all, or the majority, of these criteria are met, circulation is deemed to be adequate and staff should proceed to a standard ‘Airway to Exposure’ assessment and exclude common causes of haemodynamic compromise, such as stroke, bleeding and sepsis.
If a number of these parameters are not met, inadequate circulation can be confirmed by clinical judgement. If, by this stage, CPR has not already been implemented, it should be commenced, after adjustment of the Impella P-level to 2. The Impella should be repositioned, if necessary. If the Impella was placed in a surgical context and post-operative day <10, then chest re-opening should be performed, as per the ‘Cardiac Advanced Life Support (CALS)’ approach. The emergency deployment of VA-ECMO should also be considered.
Echocardiography
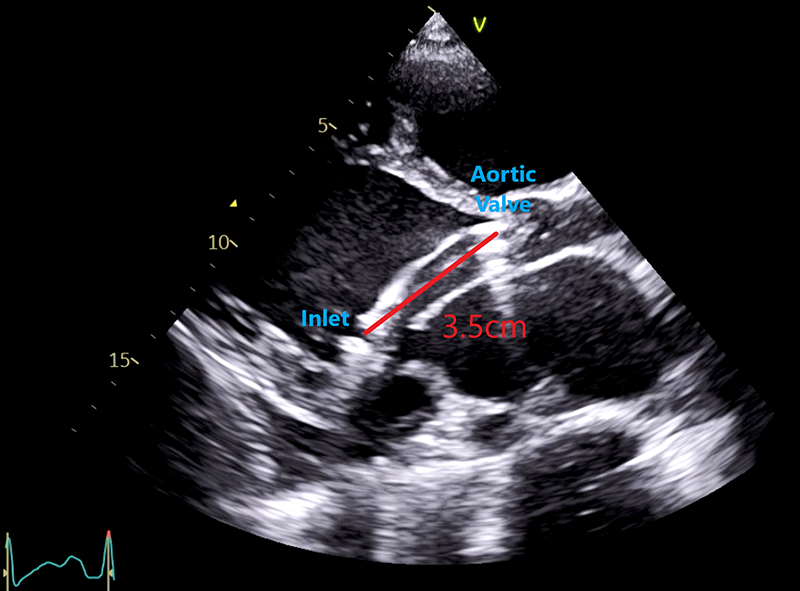
Echocardiography is indispensable in the management of an Impella recipient. The catheter position is deemed to be optimised when the distance from the inlet of the Impella to the aortic valve leaflets is 3.5 cm, with the distal end of the catheter in the central region of the left ventricle, free from obstruction by the septal wall or mitral valve apparatus (figure 2). Under conditions of unexplained clinical deterioration, echocardiography should be used to assess right ventricular function, left ventricular suction, tamponade and pump thrombosis. Echocardiography should always be used to confirm an appropriate Impella position following all resuscitative interventions. Impella therapy can also be titrated according to echocardiography parameters, such as degree of left ventricular distention, severity of mitral regurgitation and pulmonary pressures measured at differing P-levels. Complications can also be detected, such as aortic valve damage and insufficiency, aortic dissection, pericardial perforation and tamponade.
Mechanical Life Support© (MLS) course
The Impella algorithm, which details commonly encountered clinical complications, was implemented to facilitate troubleshooting by initial responders and implement appropriate remedial actions. The proposed algorithm assumes a basic level of understanding of the device, including familiarity with the connections of the catheter to the console, controls and normal operating parameters. By means of a half-day course we sought to consolidate a foundational understanding of Impella therapy.
Candidates, structure, simulator, environment
The course was advertised to all hospital staff and 14 participants from nursing, medical and allied healthcare professional backgrounds attended each course. The breadth of experience ranged from no previous Impella experience to daily exposure. The venue for the course was the hospital’s simulation suite. We used the Castle Andersen SimMon app, in combination with an Apple iPad, to display simulated vital sign parameters. A purpose-made pulsatile lumped parameter mock circulation model incorporating an Impella CP catheter was constructed to allow the simulation of representative clinical conditions during normal and abnormal device operation.
Lectures provided an introduction to advanced heart failure, temporary and long-term mechanical support, and transplantation. Subsequently, the modus operandi of Impella was demonstrated to groups of five attendees using the mock circulation model. These groups subsequently participated in a wide range of clinical simulations, including left ventricular suction, ventricular arrhythmia, and ventricular and aortic catheter displacement. The full day also included long-term LVAD training, as previously described,8 and extracorporeal membrane oxygenation (ECMO) training.
Feedback and assessment
Pre- and post-course multiple-choice questions (MCQs) and ongoing observational assessments were conducted to determine if attendees had gained adequate competency. Additional teaching was provided to those who needed further support after the course on a one-to-one basis. Course effectiveness was assessed according to the change in a self-assessed confidence score graded by Likert scale (1 = not confident at all to
5 = very confident) for both the Impella and ECMO course components and by means of a MCQs examination for Impella (maximum score 17), which focused on basic patient assessment, Impella components and algorithm use in clinical emergencies.
Key performance indicator
We identified a key performance indicator (KPI): the time taken for a member of staff to recognise a suction alarm causing hypotension, and respond by reducing the Impella CP P-level and administering an appropriate fluid bolus. The time taken was defined as the duration from initiation of the simulation until resolution of the problem, as described above, up to a maximum of 180 seconds, at which time the test was stopped. A simulation of an intensive-care patient room using the equipment described above was provided. Initially, each staff member was randomised to one of two groups using an online random number generator: 1 = standard practice; 2 = use of the algorithm. The instructor who selected the candidate and supervised the simulation was blinded to the randomisation until the simulation was commenced.
Statistical analysis
All numerical variables are presented as median (interquartile range, IQR). The unit of the KPI (time taken to resolve a suction event) was seconds.
Comparisons between standard practice and algorithm use, pre- and post-training confidence rating, and MCQ scores were made using the Wilcoxon rank-sum test.
The difference in KPI times between the two methods was estimated with the Hodges-Lehmann estimate and reported with 95% confidence intervals (CI) due to non-parametric distribution of the population.
The Hodges-Lehmann estimator for matched pairs is defined as the median of the set of n(n+1)/2 Walsh averages.13 More specifically, the process entails estimating the average difference in outcomes (x – y) for every possible n(n+1)/2 pair and then deriving the overall median of all averages (the Hodges-Lehmann estimator). A distribution-free confidence interval is estimated using large-sample approximation.14
The analysis was performed using the statistical software Stata version 17 (StataCorp LLC, Texas).
Results
A total of 53 candidates attended the MLS course and completed the pre- and post-course MCQ and confidence assessments. Following training, the median confidence scores assessing the half-day course, including ECMO and Impella, increased from 2 (IQR 2 to 3) to 4 (IQR 4 to 4) (p<0.0001). Theoretical knowledge of the Impella, assessed by median MCQ score, increased from 12 (IQR 10 to 13) to 13 (IQR 12 to 14) (p<0.0001) (table 1).
Table 1. Mechanical Life Support course confidence and multiple-choice examination results. Key performance indicator results
| Number | Percentile | |||||
|---|---|---|---|---|---|---|
| 50th | 25th | 75th | p value | |||
| Mechanical Life Support course | Confidence (Likert 1–5 score) | |||||
| Pre-course | 53 | 2 | 2 | 3 | ||
| Post-course | 53 | 4 | 4 | 4 | <0.0001 | |
| Multiple-choice questions (max 17 score) | ||||||
| Pre-course | 53 | 12 | 10 | 12 | ||
| Post-course | 53 | 13 | 13 | 14 | <0.0001 | |
| Key performance indicator | Timing to resolved problem (seconds) | |||||
| Baseline | 18 | 93 | 82 | 196 | ||
| Algorithm used | 18 | 45.5 | 35 | 49 | ||
The KPI was assessed in 36 participants working in our intensive-care unit, with 18 in each arm. Use of the Impella algorithm reduced the time taken to identify and resolve a suction event from a median of 93 (IQR 82 to 169) to 45.5 (IQR 35 to 49) seconds. The mean reduction in the time taken to resolve the suction event in the Impella algorithm group was 53 seconds (95%CI 36 to 99, p=0.0003). Four of the candidates (22%) failed to perform the correct intervention in the standard practice group and none in the algorithm group, in the time allotted.
Discussion
We identified an urgent unmet clinical need for comprehensive guidelines to assist clinicians in managing precipitous haemodynamic decompensation in patients supported with Impella LVADs. However, we encountered a number of challenges in the development of the algorithm, which aims to fulfil this unmet need. Foremost, there is very limited published guidance for clinicians faced with an Impella/cardiac arrest scenario, not least, the most basic consideration, i.e. the recognition of cardiac arrest per se. In spite of the ETCO2 value being a reasonable indicator of the adequacy of circulation, not all Impella recipients are intubated at time of arrest.15 Thus, we included a MAP value of <30 mmHg as an unequivocal marker of cardiac arrest, through expert consensus at our centre, until a more appropriate marker is identified.
Second, though we have attempted to simplify the resolution of commonly encountered problems in Impella recipients, the clinical management of such patients may be complex, necessitating a high level of staff skill and experience. Notably, malpositioning of the Impella can only safely be managed by experts. Nevertheless, we prioritised the early recognition of malposition of the Impella by all staff, including more junior members, in order to facilitate timely resolution of the issue.
Aside from the issues addressed by the algorithm, there are other potential causes of sudden haemodynamic compromise. The maintenance of purge fluid pressure and flow is essential to prevent blood ingress between the rotating impeller and its catheter in order to prevent thrombus formation, motor seizure and loss of circulatory support. Such a situation is a clinical emergency. However, purge system failure is a relatively infrequent occurrence subject to adherence to the protocol of: infusion bag replacement, inspection of the purge system and regular inspection of the extracorporeal Impella components. Irrespectively, purge system failure is a life-threatening event and demands timely resolution.
In spite of the demonstrated effectiveness of the MLS training course, it is noteworthy that self-assessed staff confidence scores and multiple-choice examination scores were comparatively low prior to training, even in experienced staff. This highlights the importance of considering the meaning of competency in the clinical setting, particularly in relation to complex medical devices, and organising adequate training.
Limitations of this initiative include a single-centre study design and inadequate evidence to provide prescriptive guidance in advanced life support involving Impella use. We sought to overcome these limitations by extensive multi-disciplinary team engagement and repeated evaluation of the algorithm. While the evidence-base remains limited, we believe that the algorithm fulfils an important, previously unmet, clinical need by facilitating the prompt recognition of both technical and medical issues and the timely implementation of remedial actions
Key messages
- Limited guidance is available to support the resuscitation of patients with the Impella CP and 5.0 devices during episodes of cardiac arrest or life-threatening events that can result in haemodynamic decompensation
- We present an evidence-based resuscitation algorithm that provides both technical and medical guidance to clinicians responding to life-threatening events in Impella recipients
- While the evidence-base remains limited, we believe that the algorithm fulfils an important, previously unmet, clinical need by facilitating the prompt recognition of both technical and medical issues, and the timely implementation of remedial actions
Conflicts of interest
WA: Abbott/Medtronic/Abiomed educational grant. SP: Abiomed honoraria. AR: Abiomed honoraria. VP: education and fellowship grant and Abiomed honoraria. KK, AW-Z, AKHC, WB, CTB, JD: none declared.
Funding
None.
Study approval
Ethical approval was not required for the algorithm development and the implementation of an educational course.
Acknowledgement
We would like to thank the medical, nursing and allied healthcare professionals who contributed to the development of this algorithm.
References
1. Amin AP, Spertus JA, Curtis JP et al. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation 2020;141:273–84. https://doi.org/10.1161/CIRCULATIONAHA.119.044007
2. Panuccio G, Neri G, Macrì LM, Salerno N, de Rosa S, Torella D. Use of Impella device in cardiogenic shock and its clinical outcomes: a systematic review and meta-analysis. Int J Cardiol Heart Vasc 2022;40:101007. https://doi.org/10.1016/j.ijcha.2022.101007
3. Affas ZR, Touza GG, Affas S. A meta-analysis comparing venoarterial (VA) extracorporeal membrane oxygenation (ECMO) to Impella for acute right ventricle failure. Cureus 2021;13:e19622. https://doi.org/10.7759/cureus.19622
4. Schrage B, Becher PM, Bernhardt A et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation 2020;142:2095–106. https://doi.org/10.1161/CIRCULATIONAHA.120.048792
5. Abiomed. Impella ventricular support systems for use during cardiogenic shock and high-risk PCI: Impella 2.5TM, Impella 5.0TM, Impella LDTM, and Impella CP® (Shock) Impella 2.5TM and Impella CP® (HRPCI). Instructions for use and clinical reference manual (United States only). Danvers, MA: Abiomed, 2017. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf14/p140003s018d.pdf [accessed 19 September 2022].
6. Abiomed. Impella Connect. Available at: https://impellaconnect.com/login [accessed 16 October 2022].
7. Bowles CT, Hards R, Wrightson N et al. Algorithms to guide ambulance clinicians in the management of emergencies in patients with implanted rotary left ventricular assist devices. Emerg Med J 2017;34:842–9. https://doi.org/10.1136/emermed-2016-206172
8. Akhtar W, Gamble B, Kiff K et al. Mechanical life support algorithm developed by simulation for inpatient emergency management of recipients of implantable left ventricular assist devices. Resusc Plus 2022;10:100254. https://doi.org/10.1016/j.resplu.2022.100254
9. Monteagudo-Vela M, Panoulas V, García-Saez D, de Robertis F, Stock U, Simon AR. Outcomes of heart transplantation in patients bridged with Impella 5.0: comparison with native chest transplanted patients without preoperative mechanical circulatory support. Artif Organs 2021;45:254–62. https://doi.org/10.1111/aor.13816
10. Peberdy MA, Gluck JA, Ornato JP et al. Cardiopulmonary resuscitation in adults and children with mechanical circulatory support: a scientific statement from the American Heart Association. Circulation 2017;135:e1115–e1134. https://doi.org/10.1161/CIR.0000000000000504
11. Aggarwal S, Bannon S. Displacement of impella post chest compressions. Heart Views 2014;15:127–8. https://doi.org/10.4103/1995-705X.151090
12. Peritz DC, Linstroth L, Selzman CH, Gilbert EM. Left ventricular perforation after Impella® placement in a patient with cardiogenic shock. Catheter Cardiovasc Interv 2018;91:894–6. https://doi.org/10.1002/ccd.27329
13. Walsh JE. Applications of some significance tests for the median which are valid under very general conditions. J Am Stat Assoc 1949;44:342–55. https://doi.org/10.1080/01621459.1949.10483311
14. Hollander M, Wolfe DA, Chicken E. Nonparametric Statistical Methods (3rd ed). Hoboken, NJ: John Wiley and Sons, 2014. https://doi.org/10.1002/9781119196037
15. Paiva EF, Paxton JH, O’Neil BJ. The use of end-tidal carbon dioxide (ETCO2) measurement to guide management of cardiac arrest: a systematic review. Resuscitation 2018;123:1–7. https://doi.org/10.1016/j.resuscitation.2017.12.003
