Transcatheter edge-to-edge repair (TEER) was first performed in 2003, and is now established across the developed world as an effective, minimally invasive treatment option for patients with mitral regurgitation (MR). Multiple large registries have established the efficacy of mitral TEER in patients with primary or degenerative MR in whom surgery is considered prohibitive or high risk, while ongoing randomised-controlled trials will determine its role in younger and lower-risk patients. In patients with secondary or functional MR, in whom mitral valve surgery is not routinely recommended, the pivotal COAPT trial showed a profound reduction in both mortality and heart failure hospitalisation in carefully selected patients.
NHS England approved the routine commissioning of mitral TEER in 2019, and following a substantial delay, due in large part to the COVID pandemic, the procedure is now widely available across the UK. This review article describes the TEER procedure, currently available devices, the underlying evidence base, and the key facts needed for clinicians to understand who, how, and where to refer patients for consideration of mitral TEER. The emerging role of TEER in patients with severe symptomatic tricuspid regurgitation is also considered.
Background
Transcatheter edge-to-edge repair (TEER) is a percutaneous catheter-based technique that aims to replicate the Alfieri stitch, a surgical technique in which a suture is applied at the site of the regurgitant jet between the facing free margins of the anterior and posterior leaflets of the mitral valve (MV), creating a double orifice valve without residual prolapse of one or both leaflets. After a 10-year wait for funding, TEER has now finally become widely available across the National Health Service (NHS). This article will provide an overview of the TEER procedure, describe currently available technology, and outline who and how to refer for edge-to-edge repair, including the required imaging.
Nomenclature
Transcatheter edge-to-edge repair has evolved as the preferred terminology for this procedure, and may be applied to its use in both the mitral and tricuspid valves. However, percutaneous MV leaflet repair has also been widely employed, including in both the NHS England (NHSE) commissioning statement, and National Institute for Health and Care Excellence (NICE) guidance.1,2 The term mitral clip was previously commonly used in reference to the original, and previously only, available device. While all of these terms will still often be heard in clinical practice, transcatheter edge-to-edge repair is now accepted worldwide as the standard nomenclature.
Current status of TEER in the UK
The number of TEER procedures performed globally has increased rapidly over the past 15 years, with more than 10,000 patients treated annually in the US alone.3 In contrast, TEER has been slow to take off in the UK. Europe-wide data from 2019 showed that the number of procedures performed in the UK was just 100, equating to 1.5 per million population, fewer than any other Western European country (figure 1). This historical under-provision is attributable to a lack of funding by NHSE and the devolved authorities. Following completion of a limited observational assessment of TEER in just three centres in England, commissioning approval of TEER for the treatment of degenerative MR by NHSE was finally given in July 2019.1
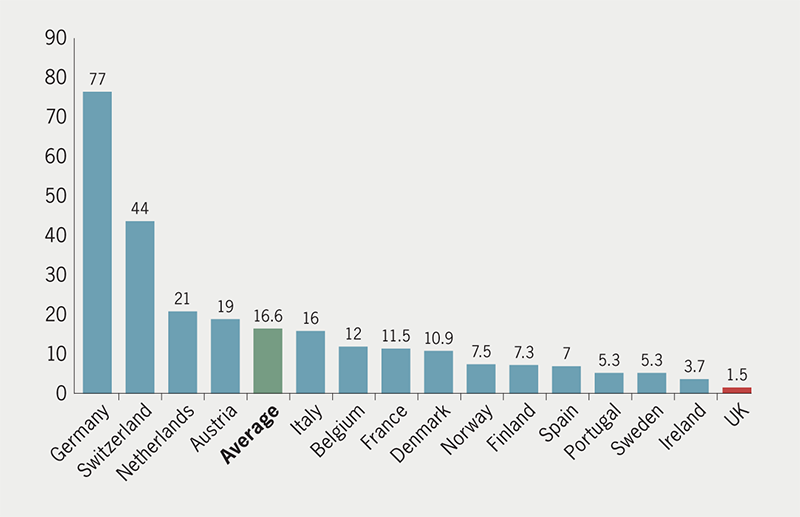
Table 1. Transcatheter edge-to-edge repair (TEER) centres in the UK
| Region | Centre | Lead clinician |
|---|---|---|
| London | Barts | Mike Mullen |
| Brompton / Harefield | Rob Smith | |
| Hammersmith | Mike Bellamy | |
| King’s | Jonathan Byrne | |
| St Thomas’s | Tiffany Patterson | |
| South East | Brighton | David Hildick-Smith |
| Oxford | Sam Dawkins | |
| Southampton | Michael Mahmoudi | |
| South West | Bristol | Mark Turner |
| East | Papworth | Patrick Calvert |
| Midlands | QE Birmingham | Sagar Doshi |
| Glenfield, Leicester | Jan Kovac | |
| Royal Stoke | Adrian Large | |
| Wolverhampton | Saib Khogali | |
| Nottingham | Will Smith | |
| North West | Manchester | Mamta Buch |
| Liverpool | Clare Appleby | |
| Yorkshire and North East | Leeds | Chris Malkin |
| Middlesbrough | Paul Williams | |
| Newcastle | Mohaned Egred | |
| Scotland | Glasgow | Angela Ghattas |
| Edinburgh | David Northridge | |
| Wales | Cardiff | Richard Anderson |
| Northern Ireland | Belfast | Colm Owens |
The subsequent planned roll-out of TEER was delayed further by the COVID pandemic. However, across the course of 2021 and 2022, additional specialist centres across the country have gradually received approval from NHSE to perform TEER. This key minimally invasive intervention for MV disease is now available to patients across England and the devolved nations. Table 1 shows the centres currently performing TEER in the UK.
The mitral TEER procedure
TEER is performed under general anaesthesia (GA) and is guided by trans-oesophageal echocardiography (TOE) and fluoroscopy.
Percutaneous ultrasound-guided femoral venous access is performed for introduction of the large-bore delivery sheath, with catheter-based pre-closure using suture-based devices. The procedure requires a targeted TOE-guided high posterior trans-septal puncture to enable sufficient height above the MV to perform grasping, usually between 4 and 5 cm. The trans-septal sheath is advanced into the left atrium (LA) and exchanged for a stiff support wire. The device guiding catheter is advanced into the LA to enable delivery of the clip or implant system, which is then steered towards the MV. The precise strategy of edge-to-edge repair will depend on the nature and extent of the mitral regurgitation (MR). MR reduction is measured in real-time by TOE, and together with monitoring of LA pressure and trans-mitral gradient, will influence the decision to move or deploy the implant. Latest iterations of the currently available devices allow optimisation of individual leaflet insertion to maximise MR reduction and minimise the risk of leaflet detachment after deployment. After the clip is deployed, the reduction in regurgitation, together with the MV gradient, is assessed to determine the need for further implants. At the end of the procedure the guiding catheter is retracted into the right atrium. In general, iatrogenic atrial septal defects are not closed percutaneously at the time of the procedure. This can be considered, however, where there is evidence of right-to-left shunting or of more extensive disruption of the septum.
Currently available TEER devices
There are currently two CE-marked TEER devices available for use in the UK. The MitraClip system (Abbott Vascular) is a two-armed cobalt chromium device with a polyester covering. The fourth-generation MitraClip (Gen 4) is available in four different sizes, enabling the procedure to be tailored to the pathology being treated. Gen 4 also enables independent leaflet grasping, allowing the operator to capture the leaflets sequentially and to optimise leaflets after initial grasping.
The PASCAL system (Edwards Lifesciences) boasts broader paddles to facilitate leaflet capture, and a central spacer that functions both to fill the regurgitant orifice and reduce the risk of iatrogenic mitral stenosis. The PASCAL introduced the concept of independent grasping, whereby leaflets can be captured separately. The unique ability of the device to elongate facilitates atraumatic removal of the implant and guiding catheter from the left ventricle if required.
TEER for degenerative MR
Primary or degenerative mitral regurgitation (DMR) results from an intrinsic abnormality of the valve, including flail and prolapsing segments or myxomatous degeneration, in contrast to secondary or functional MR, which results from changes in left ventricular or left atrial volume, resulting in annular dilatation, leaflet tethering and restriction.
Evidence-base
The EVEREST (Endovascular Valve Edge-to-Edge REpair STudy) trial was the first study to evaluate the effectiveness of TEER compared with surgery.4 The study recruited 279 patients with predominantly DMR (70%), who were randomised 2:1 to TEER with the MitraClip system or conventional surgery. The primary end point of freedom from death, re-intervention and recurrent MR was higher in the MitraClip arm (73% vs. 55%), driven by the need for MV surgery in a fifth of TEER patients, compared with 2% in the surgical arm. MitraClip was safer than surgery, with a major adverse event rate of 15% at 30 days compared with 48% in the surgical arm, driven largely by a reduced need for blood transfusion. Symptoms were improved in both groups. The durability of TEER was confirmed in the five-year follow-up, with no difference in re-intervention rates between surgery and TEER beyond the first six months.5
Insight into more contemporary outcomes following TEER comes from the US Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) registry. Almost 34,000 procedures were undertaken over a six-year period (2014 to 2020) to treat predominantly DMR (71%). There was a very high rate of procedural success (98%), and a substantial reduction in MR to moderate or less in 91%. In-hospital mortality was 2.2% and conversion to open surgery occurred in <0.5%.3 More recently, the CLASP IID (Edwards PASCAL Transcatheter Valve Repair System Pivotal Clinical Trial) study evaluated the effectiveness of the PASCAL system in a randomised comparison with MitraClip in 180 patients with DMR. No major differences were seen between cohorts. MR was reduced to mild or trace in almost 90% of patients treated with either technology, a reflection of improved operator experience and device design.6
Who should be referred?
Current guidelines recommend TEER for those patients with a contraindication to surgery or who are at high operative risk, and have suitable anatomy.7 Degenerative pathology involving the central aspect of the valve (P2 or A2 flail or prolapse) with good leaflet quality is considered ideal, along with a relatively short flail width (<10 mm) and flail gap (<15 mm). In reality, there are a broad range of degenerative pathologies that can be treated successfully with TEER, including large flail segments, non-central/commissural prolapse, multi-segment prolapse, and Barlow’s disease. A small number of contraindications to TEER do exist, particularly extensive leaflet calcification in the grasping zone, a small valve area (<3.5 cm), and rheumatic pathology. Anatomical selection criteria are summarised in table 2.
Table 2. Anatomical selection criteria for mitral TEER in degenerative mitral regurgitation (MR)
| Favourable anatomy | Less favourable | Contraindicated |
|---|---|---|
|
|
|
| Key: MVA = mitral valve area | ||
TEER can also be used successfully in patients with acute ischaemic mitral regurgitation post-myocardial infarction, and is often the only therapeutic treatment option for this cohort.8
Future studies
Two pivotal randomised-controlled trials are currently in recruitment. The REPAIR MR (Percutaneous MitraClip Device or Surgical Mitral Valve Repair in Patients With Primary Mitral Regurgitation Who Are Candidates for Surgery) trial will randomise patients at moderate surgical risk to TEER or conventional surgery, while the PRIMARY (Percutaneous or Surgical Repair In Mitral Prolapse And Regurgitation for ≥65 Year-olds) study will include all patients over the age of 65, regardless of risk. The results will further define the role of TEER in the treatment of patients with DMR.
TEER for functional MR
Until recently, therapeutic options for patients with secondary or functional mitral regurgitation (FMR) have largely focused on pharmacological and cardiac resynchronisation therapy, with surgical intervention reserved for patients with concomitant coronary artery disease. Isolated MV surgery for this population of patients is rarely recommended in current guidelines due to high procedural risk and no evidence of a mortality benefit.7 TEER offers a new therapeutic option, aiming to reduce heart failure hospitalisation and improve clinical and quality-of-life outcomes.
Evidence-base
There are two randomised trials of TEER for patients with FMR. The COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) study enrolled 614 subjects randomised 1:1 to TEER with MitraClip, or to guideline-directed medical therapy (GDMT).9 After 24 months there was a significant mortality reduction in the TEER group (29.1% vs. 46.1%, number needed to treat [NNT] to prevent one death 5.9). There was a similarly striking effect on heart failure rehospitalisation, with NNT to prevent one rehospitalisation 3.1.10 In contrast, the MITRA-FR (Multicentre Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients With Severe Secondary Mitral Regurgitation) study showed no significant difference between TEER and control for both all-cause mortality and heart failure rehospitalisation.11
Important differences in the two trials are likely to explain such contrasting results, and help guide patient selection for TEER in FMR. Patients enrolled to COAPT had more severe MR (effective regurgitant orifice area [EROA] 41 ± 15 vs. 31 ± 10 mm) and less left ventricular (LV) dilatation (LV end diastolic volume 101 ± 34 vs. 135 ± 35 ml/m2) than those in MITRA-FR. MR reduction was greater in COAPT, with more patients with ≤2+ MR after TEER (95% vs. 83%). The approach to management of GDMT was also different. MITRA-FR followed a more ‘real-world’ approach, with patients on their own heart failure medications at baseline, whereas COAPT mandated that patients in both groups were confirmed to be on maximally tolerated GDMT before randomisation.
A key concept that helps to explain the differing response of patients in the two trials is that of proportionality of MR, where the role of ventricular dilatation is considered in relation to severity of MR.12 For a given LV volume and regurgitation fraction, the expected EROA can be predicted. Where MR is proportionate to the degree of LV dilatation, it may be more likely to respond to device and pharmacological therapy. Where MR is disproportionately severe (EROA greater than predicted), patients are more likely to benefit from TEER.
Who should be referred?
In keeping with the evidence-base and international guidelines, patients who fit the COAPT inclusion criteria should be considered for TEER. Referral should be made for patients with severe MR due to LV dysfunction in whom intrusive symptoms persist despite appropriately up-titrated GDMT. Patients with FMR due solely to atrial dilatation, usually in the context of longstanding atrial fibrillation, may also be successfully treated with TEER, although evidence in this group is lacking.
Imaging the MV for TEER
Table 3. Key information required from the pre-procedural echocardiogram
| Valve morphology |
|---|
| DMR vs. FMR |
| MR jet – one significant jet? More significant jets? |
| Flail width <15 mm and flail gap <10 mm |
| Leaflet mobility length >10 mm |
| Calcification |
| Calcification |
| Severity |
| Integrated assessment |
| Qualitative |
| Semi-quantitative |
| Quantitative |
| Other |
| Mitral valve area >4 cm2 |
| Left atrial size >35 mm |
| Trans-septal puncture height >3.5 cm |
| Key: DMR = degenerative mitral regurgitation; FMR = functional mitral regurgitation; MR = mitral regurgitation |
Echocardiography is the key imaging modality for the pre-procedural, procedural, and post-procedural assessment of patients undergoing TEER. Both transthoracic echocardiography (TTE) and TOE should be performed, including bi-plane and 3D imaging.
The main purpose of pre-procedural imaging is to determine if patients have suitable MV anatomy for TEER, and should include assessment of MV morphology, MR severity, and anatomical information required for the TEER procedure (table 3).
MV morphology
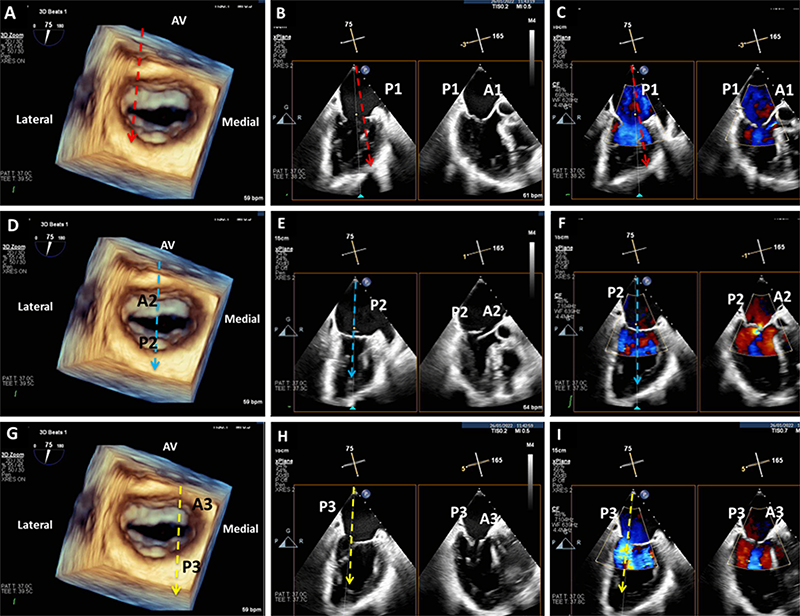
The key views to assess structural suitability for TEER are the bi-commissural, which depicts the medial-lateral aspect of the MV, and the long axis, which shows the anterior-posterior aspect.
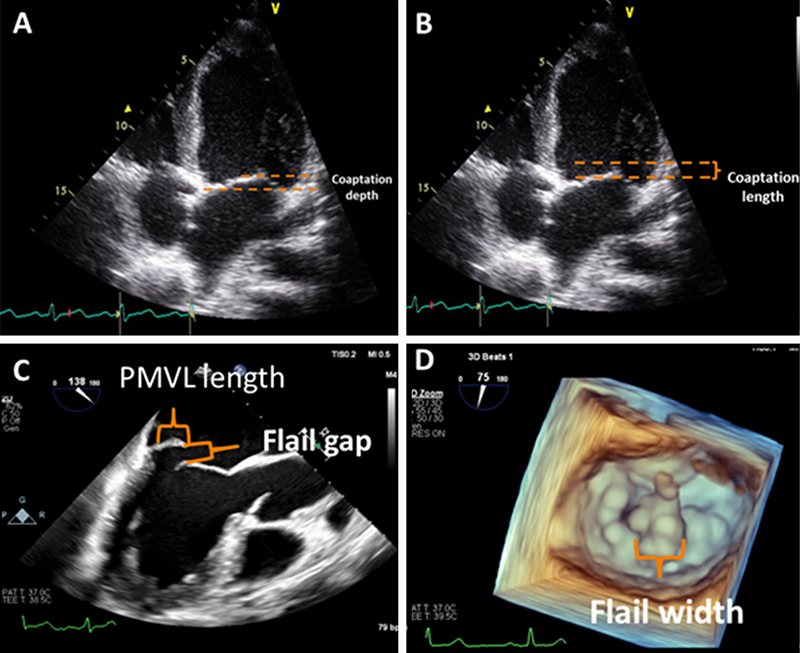
A 3D matrix transducer should be used to perform simultaneous multi-plane imaging. An optimised bi-commissural view should first be obtained as the primary image, following which, use of the multi-plane mode will allow an orthogonal long-axis view to be visualised simultaneously as a secondary image. This allows a step-by-step assessment of MV morphology using MV segmental analysis, making it easier to assess the specific location of the regurgitation jet and mechanism of valve pathology (figure 2). By sweeping the cursor along the whole line of coaptation, with and without colour doppler, a detailed structural assessment of all MV scallops can be obtained. In addition to MV segmental analysis, a 3D ‘en-face’ view should be obtained to define leaflet morphology (figure 2A). This is also a key view used to guide clip positioning during the TEER procedure. Morphological assessment should also include measurement of the coaptation depth and length in cases of FMR, and in DMR cases the flail gap and width (figure 3).
MR severity
A comprehensive integrated assessment of MR severity using qualitative, semi-quantitative and quantitative parameters is recommended. In patients with FMR, assessment of severity is more challenging due to the non-circular regurgitant orifice area, as well as the dynamic nature of MR, which will vary with haemodynamic status; regurgitation fraction is the most physiological and reliable parameter in these patients.
Assessment of anatomy specific to the TEER procedure
Mitral valve area (MVA) should be measured to determine the risk of mitral stenosis after TEER, either by planimetry at the tips of the MV leaflets in the TTE short-axis view, or more accurately by 3D planimetry on TOE. Assessment of the inter-atrial septum and left atrial size should be performed to determine the feasibility of trans-septal puncture, including available height above the MV for the TEER procedure.
Finally, calcification of the leaflets and subvalvular apparatus should be identified, since excessive calcification, particularly in the grasping zone, may preclude TEER.
Tricuspid edge-to-edge repair
Tricuspid regurgitation (TR) is common, and associated with significant morbidity and mortality, but is generally poorly investigated and treated.13 Moreover, open heart surgery for TR is rarely performed in isolation and has been associated with poor results.14
The increasing awareness of effective TEER techniques for the MV generated the realisation that a similar therapy may have a use with the tricuspid valve, even with this valve’s more complex anatomy.15
Evidence-base
Registry data, initially with the MitraClip device, and subsequently with the more tailored PASCAL and TriClip (Abbott Vascular) systems, have consistently shown tricuspid TEER (tTEER) to be very safe, with low mortality and morbidity, and effective, with sustained clinical benefit at one year.15–19 The first randomised trial of tTEER, the TRILUMINATE (Trial to Evaluate Cardiovascular Outcomes in Patients Treated With the Tricuspid Valve Repair System) study, was published in March 2023.20 In 350 patients randomised 1:1 to tTEER versus medical therapy, tTEER was safe (98.3% freedom from major adverse events at 30 days), effective in reducing TR (87.0% ≤moderate TR at 30 days vs. 4.0% control), and significantly improved quality of life at 12 months compared with medical therapy (Kansas City Cardiomyopathy Questionnaire [KCCQ] quality-of-life score increase 12.3 ± 1.8 vs. 0.6 ± 1.8, p<0.0001). However, there was no impact on death, tricuspid valve surgery, or hospitalisation for heart failure.
Case selection
Selection of patients for possible tTEER should include detailed imaging, including TOE, to establish the aetiology of TR, the precise location of the regurgitant jet, the morphology of the valve leaflets, and the coaptation gap. Atrial dilatation due to permanent atrial fibrillation, and pacing-lead induced TR, are two of the more common TR aetiologies – both are amenable to tTEER.
Patients with severe TR often have multiple comorbidities, and establishing the extent to which the TR is responsible for the overall symptom burden can be challenging. However, patients with refractory symptoms of breathlessness or right heart failure with severe TR should be referred to a specialised valve multi-disciplinary team (MDT). Although tTEER is currently not commissioned in the UK, an increasing number of centres are already performing the procedure with promising early results.
Conclusion
Mitral TEER is a safe, low-risk, and effective therapeutic option for patients with DMR who are at prohibitive or high-risk for MV surgery due to age and/or comorbidities, and for those with disproportionate FMR with symptoms refractory to medical therapy. Mitral TEER is now, finally, widely available across the NHS for the significant cohort of patients who meet these criteria, while the use of TEER for the under-recognised but common problem of severe symptomatic tricuspid regurgitation is developing. Potentially suitable patients should be referred to the specialist valve MDTs in place at all UK TEER centres.
Key messages
- Mitral valve transcatheter edge-to-edge repair (TEER), formerly known as percutaneous mitral valve leaflet repair or mitral clip, is now commissioned by NHS England and the devolved authorities, and is routinely available in 24 cardiac surgical centres across the UK
- Referral for mitral TEER should be considered in patients with primary or degenerative mitral regurgitation who are at high or prohibitive risk for mitral valve surgery, and in patients with secondary or functional mitral regurgitation who remain symptomatic despite optimal medical therapy
- Tricuspid valve TEER is emerging as a safe and effective treatment option for the large and often unrecognised cohort of patients with severe tricuspid regurgitation, for whom no good surgical option exists
- This review describes the current status of the TEER procedure, considers which patients should be referred for intervention, reviews the pre-procedural imaging required when considering mitral TEER, and outlines the evidence for the application of TEER to the tricuspid valve
Conflicts of interest
DJB is a Consultant and Proctor for Abbott Vascular, Edwards Lifesciences, and Medtronic. SD is a Consultant and Proctor for Abbott Vascular and Edwards Lifesciences. RS is a Consultant and Proctor for Abbott Vascular. JB is a Consultant and Proctor for Abbott Vascular and Edwards Lifesciences. PAM is a Consultant and Proctor for Abbott Vascular and Edwards Lifesciences. DS none declared.
Funding
Funding for this article was provided by the Valve for Life programme, with a grant obtained from the European Association of Percutaneous Cardiovascular Interventions.
References
1. NHS England. Clinical commissioning policy: percutaneous mitral valve leaflet repair for primary degenerative mitral regurgitation in adults. London: NHS England, July 2019. Available from: https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2019/07/Clinical-Commissioning-Policy_Percutaneous-mitral-valve-leaflet-repair-for-primary-degenerative-mitral-regurgi.pdf
2. National Institute for Health and Care Excellence. Percutaneous mitral valve leaflet repair for mitral regurgitation. IPG649. London: NICE, 2019. Available from: https://www.nice.org.uk/guidance/ipg649
3. Mack M, Carroll JD, Thourani V et al. Transcatheter mitral valve therapy in the United States: a report from the STS-ACC TVT registry. J Am Coll Cardiol 2021;78:2326–53. https://doi.org/10.1016/j.jacc.2021.07.058
4. Feldman T, Foster E, Glower DD et al.; EVEREST II Investigators. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395–406. https://doi.org/10.1056/NEJMoa1009355
5. Feldman T, Kar S, Elmariah S et al.; EVEREST II Investigators. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol 2015;66:2844–54. https://doi.org/10.1016/j.jacc.2015.10.018
6. Lim DS, Smith RL, Gillam LD et al. Randomized comparison of transcatheter edge-to-edge repair for degenerative mitral regurgitation in prohibitive surgical risk patients. JACC Cardiovasc Interv 2022;15:2523–36. https://doi.org/10.1016/j.jcin.2022.09.005
7. Vahanian A, Beyersdorf F, Praz F et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561–632. https://doi.org/10.1093/eurheartj/ehab395
8. Farwati M, Saad AM, Abushouk AI et al. Short-term outcomes following urgent transcatheter edge-to-edge repair with MitraClip in cardiogenic shock: a population-based analysis. JACC Cardiovasc Interv 2021;14:2077–8. https://doi.org/10.1016/j.jcin.2021.04.052
9. Stone GW, Lindenfeld J, Abraham WT et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379:2307–18. https://doi.org/10.1056/NEJMoa1806640
10. Giustino G, Camaj A, Kapadia SR et al. Hospitalizations and mortality in patients with secondary mitral regurgitation and heart failure. J Am Coll Cardiol 2022;80:1857–68. https://doi.org/10.1016/j.jacc.2022.08.803
11. Obadia JF, Messika-Zeitoun D, Leurent G et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018;379:2297–306. https://doi.org/10.1056/NEJMoa1805374
12. Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation: a new conceptual framework that reconciles the results of the MITRA-FR and COAPT trials. JACC Cardiovasc Imaging 2019;12:353–62. https://doi.org/10.1016/j.jcmg.2018.11.006
13. Wang N, Fulcher J, Abeysuriya N et al. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: a systematic review and meta-analysis. Eur Heart J 2019;40:476–84. https://doi.org/10.1093/eurheartj/ehy641
14. Kawsara A, Alqahtani F, Nkomo VT et al. Determinants of morbidity and mortality associated with isolated tricuspid valve surgery. J Am Heart Assoc 2021;10:e018417. https://doi.org/10.1161/JAHA.120.018417
15. Nickenig G, Kowalski M, Hausleiter J et al. Transcatheter treatment of severe tricuspid regurgitation with the edge-to-edge MitraClip technique. Circulation 2017;135:1802–14. https://doi.org/10.1161/CIRCULATIONAHA.116.024848
16. Mehr M, Taramasso M, Besler C et al. 1-year outcomes after edge-to-edge valve repair for symptomatic tricuspid regurgitation. J Am Coll Cardiol Interv 2019;12:1451–61. https://doi.org/10.1016/j.jacc.2019.09.028
17. Aurich M, Volz MJ, Mereles D et al. Initial experience with the PASCAL ace implant system for treatment of severe tricuspid regurgitation. Circ Cardiovasc Interv 2021;14:e010770. https://doi.org/10.1161/CIRCINTERVENTIONS.121.010770
18. Lurz P, von Bardeleben RS, Weber M et al. Transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol 2021;77:229–39. https://doi.org/10.1016/j.jacc.2020.11.038
19. Taramasso M, Benfari G, van der Bijl P et al. Transcatheter versus medical treatment of patients with symptomatic severe tricuspid regurgitation. J Am Coll Cardiol 2019;74:2998–3008. https://doi.org/10.1016/j.jacc.2019.09.028
20. Sorajja P, Whisenant B, Hamid N et al. Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med 2023;388:1833–42. https://doi.org/10.1056/NEJMoa2300525
