Stroke is a major cause of mortality, morbidity and economic burden. Strokes can be thrombotic, embolic or haemorrhagic. The key risk factor for cardioembolic stroke is atrial fibrillation or flutter, and oral anticoagulation (OAC) is recommended in all but the lowest-risk patients with evidence of these arrhythmias. Risk factors for thrombotic stroke overlap strongly with those for other atherosclerotic cardiovascular diseases (ASCVDs). Antiplatelet therapy (APT) should be considered in patients with established ASCVD to reduce risk of cardiovascular events, including stroke. Intensification from single to dual APT or a combination of APT with low-dose OAC can reduce ischaemic stroke risk further, but increases bleeding risk. Blood pressure and lipid profile should be controlled appropriately to guideline targets. In patients with diabetes, good glycaemic control can reduce stroke risk. Inflammation is another emerging target for stroke prevention. Overall, comprehensive assessment and pharmacological modification of risk factors are central to stroke prevention.
Introduction

Stroke is defined as an acute neurological deficit of cerebrovascular origin lasting longer than 24 hours. In the UK each year, stroke affects approximately 100,000 people, is a leading cause of mortality, causing over 30,000 deaths in 2020, and is a significant contributor to severe disability.1 Caring for patients with stroke in the UK costs approximately £2.5 million each year and leads to significant production losses. Clearly, preventing stroke has many benefits.
Strokes can be ischaemic (85%), where tissue damage is due to occlusion of blood supply, or haemorrhagic (15%), due to a ruptured vessel.2 Ischaemic stroke can be further divided. Thrombotic strokes manifest from cerebral artery atherosclerotic plaque rupture or erosion, leading to activation of platelets and the coagulation cascade, though platelet activation appears to be the dominant mechanism. Embolic stroke typically occurs when a thrombus formed at a distant site breaks loose and occludes a cerebral artery. This may be due to an arterial thrombus formed on plaque within the aortic arch or carotid/vertebral arteries, again platelet activation being the most prominent mechanism. Embolism can also result from intracardiac thrombosis (cardioembolism), most commonly originating in the left atrial appendage, or from a venous thrombus travelling through an interatrial connection, such as a patent foramen ovale. In these forms of embolic stroke, activation of the coagulation cascade, rather than platelets, contributes most to the pathogenesis. Risk factors for thrombotic stroke, such as hypertension, smoking, hypercholesterolaemia, diabetes mellitus (DM), and chronic inflammation, overlap with those for other forms of atherosclerotic cardiovascular disease (ASCVD), such as coronary syndromes or peripheral arterial disease (PAD). The presence of atrial fibrillation (AF) or flutter (AFL) is the major risk factor for cardioembolic stroke. Personalised modification of these risk factors is, therefore, central to stroke prevention. Few trials have included stroke alone as a primary end point, with most cardiovascular prevention trials adopting a primary end point of major adverse cardiovascular events (MACE), typically defined as cardiovascular (CV) death, stroke or myocardial infarction (MI). However, insights can be gained from these data, including the cautious interpretation of secondary end point data on stroke outcomes.
Antithrombotic therapy
Given thrombosis is central to the pathogenesis of ischaemic stroke, it is rational that antithrombotic therapy is useful in its prevention. The optimal antithrombotic strategy is heavily influenced by the presence or absence of AF/AFL.
The patient with a history of AF or AFL
AF and AFL are associated with an increased risk of cardioembolic stroke, likely due to a combination of alteration in dynamics of blood flow and inflammation creating a prothrombotic atrial milieu. The significant benefit of oral anticoagulation (OAC) over antiplatelet therapy (APT) in reducing risk of stroke in AF/AFL is evidenced, for example, by a meta-analysis of 12,963 patients showing warfarin was associated with a 39% relative risk reduction in stroke.3 Where CHA2DS2-VASc score is ≥2 if male and ≥3 if female, OAC is recommended to reduce the risk of stroke, and should be considered where score is 1 or 2 in males and females, respectively.4 Non-vitamin K antagonist OACs (NOACs), such as the factor Xa inhibitors apixaban, edoxaban and rivaroxaban, and the direct thrombin inhibitor dabigatran, offer superior all-cause stroke prevention when compared with vitamin K antagonists, such as warfarin, and, therefore, should be regarded as first line, unless there is a contraindication, or in the setting of valvular AF (with at least moderate mitral stenosis), or presence of a mechanical valve prosthesis.
The patient without a history of AF or AFL
Aspirin
Full-dose OAC is not indicated for stroke prevention in patients without an additional indication, such as AF/AFL, but other forms of antithrombotic therapy should be considered. Aspirin is an inhibitor of platelet cyclo-oxygenase-1, one of a chain of enzymes responsible for thromboxane A2 (TXA2) synthesis. TXA2 is a key mediator of platelet activation. In the setting of prevention of MACE in those without ASCVD, there is little evidence that aspirin is of net benefit. A meta-analysis of six primary prevention trials, including 95,000 individuals, suggested no significant effect on reducing the risk of all-cause stroke, but a significant increase in risk of major gastrointestinal and extracranial bleeding by approximately 50%; the risks, therefore, outweighing the benefits.5
In patients with established ASCVD, aspirin has a much clearer role. In a meta-analysis of 16 secondary prevention trials, aspirin was found to significantly reduce risk of all-cause stroke (2.08% vs. 2.54% per year), despite a non-significant increase in haemorrhagic stroke.5 Aspirin 75–100 mg once daily is recommended for reducing the risk of MACE, including stroke, in patients with a history of myocardial infarction or coronary revascularisation, and, additionally, may be considered in those with imaging evidence of coronary artery disease (CAD).6
P2Y12 inhibitor monotherapy
The platelet P2Y12 receptor is central to the amplification of platelet activation. In the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial of patients with established ASCVD, the P2Y12 inhibitor clopidogrel 75 mg once daily led to a modestly and non-significantly lower rate of ischaemic stroke compared with aspirin 325 mg once daily, with a similar safety profile.7 Overall benefits of clopidogrel over aspirin were stronger in the subgroups with cerebrovascular and peripheral artery disease (PAD), and clopidogrel monotherapy is now often used in these groups in preference to aspirin. The newer P2Y12 inhibitors, prasugrel and ticagrelor, offer a more potent and reliable antiplatelet effect than clopidogrel. In the EUCLID (Examining Use of tiCagreLor In peripheral artery Disease) trial, ticagrelor 90 mg twice daily significantly reduced the risk of ischaemic stroke compared with clopidogrel 75 mg once daily, with no significant difference in major bleeding.8 However, ticagrelor did not significantly reduce the overall rate of the primary end point of MACE, so this has not been translated into guideline recommendations.
Dual antiplatelet therapy
Dual antiplatelet therapy (DAPT) is the combination of aspirin and an oral P2Y12 inhibitor. In patients with established ASCVD, though the CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance) trial did not show a significant reduction in MACE, there was a significant reduction in the secondary end point of non-fatal stroke when receiving aspirin (75–162 mg once daily) and clopidogrel (75 mg once daily) compared with aspirin alone, without significant differences in major bleeding.9 Furthermore, in patients with ASCVD and prior MI, the PEGASUS-TIMI 54 (PrEvention with TicaGrelor of SecondAry Thrombotic Events in High-RiSk Patients with Prior AcUte Coronary Syndrome – Thrombolysis In Myocardial Infarction) trial demonstrated a significant reduction in both MACE and all-cause stroke when receiving aspirin and the reversible P2Y12 inhibitor ticagrelor (60 mg twice daily) compared with aspirin alone, with an increase in major bleeding, but not fatal bleeding.10 The DAPT (Dual AntiPlatelet Therapy) study supported this trend in patients receiving aspirin and clopidogrel or prasugrel, when compared with aspirin alone.11 European Society of Cardiology (ESC) guidelines recommend DAPT in patients with chronic coronary syndromes (CCS, which include those with prior MI or stable CAD) at high ischaemic risk and low bleeding risk to reduce risk of cardiovascular events, including ischaemic stroke.6 Based on the THEMIS (Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study) trial, ticagrelor is also licensed in the US for prevention of a first MI or stroke in aspirin-treated high-risk CAD patients.12
Low-dose dual antithrombotic therapy
An alternative to DAPT for stroke prevention in high-risk patients with CCS or PAD is low-dose dual antithrombotic therapy (DATT) with aspirin (75–100 mg once daily) and 2.5 mg twice daily of the NOAC, rivaroxaban. In the COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial, DATT was associated with a significant reduction in both MACE and all-cause stroke, with a significant increase in bleeding events, but not fatal bleeding.13
Summary: choosing an antithrombotic regimen for stroke prevention
Risk factors for ischaemia and bleeding should be considered together when deciding whether to initiate antithrombotic therapy and which regimen to recommend. In those with AF/AFL and all-but-the-lowest ischaemic risk, OAC should be recommended. In those without AF/AFL but with established ASCVD, at least single APT is generally recommended, apart from in situations such as isolated asymptomatic PAD. As well as after acute coronary syndromes (ACS) or percutaneous coronary intervention (PCI), intensification of APT to DAPT or DATT should be considered in high-risk patients with CCS (figure 1) or to DATT in symptomatic PAD.2 In those with both AF/AFL and ASCVD, in general APT is not recommended alongside full-dose OAC outside of specific situations, such as recent ACS or PCI, though longer-term combination can be considered in selected patients at very high ischaemic risk and low bleeding risk.6
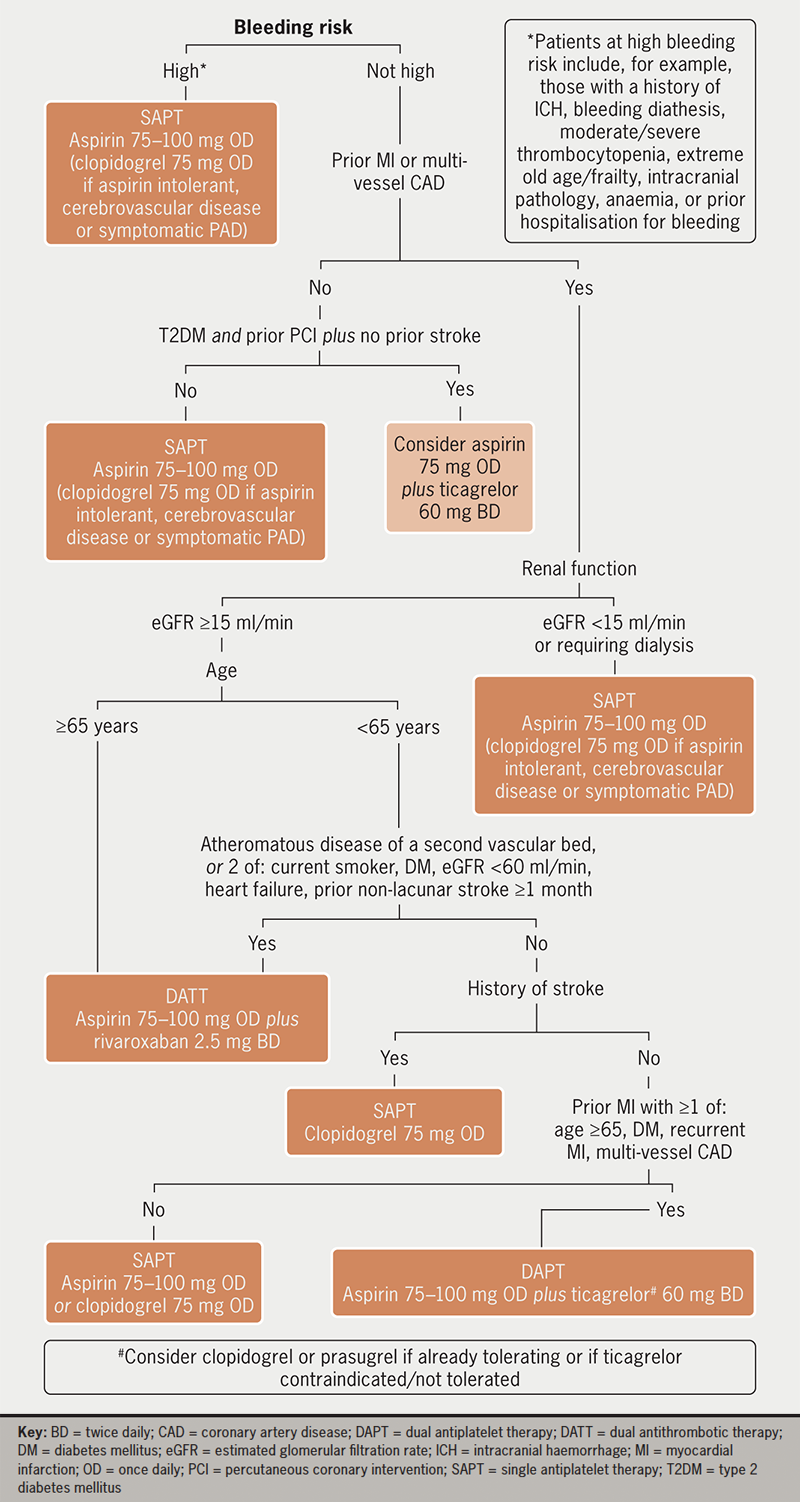
Control of blood pressure
Hypertension is a particularly important risk factor for stroke given it can contribute to both ischaemic and haemorrhagic events. It has been shown to increase risk of stroke in patients with or without ASCVD, particularly those below the age of around 70 years, with a clear relationship between level of hypertension and increase in risk. Current ESC guidelines recommend a target of <140/90 mmHg in all patients, falling to <130/80 mmHg in most patients if initial treatment is well tolerated. A systolic (SBP) target of 120–129 mmHg should be routinely aimed for in those <65 years old, and diastolic blood pressure (DBP) should be kept <80 mmHg in all hypertensive patients.14 Home or ambulatory monitoring of blood pressure can aid accurate assessment of ongoing risk and avoid overtreatment. In general, choice of antihypertensive appears to be less important than the target achieved, though renin–angiotensin–system inhibitors are generally of more benefit if there is prior MI, diabetes mellitus, chronic kidney disease (CKD) or heart failure with reduced ejection fraction, and initial regimens avoiding a beta blocker may have better overall impact on clinical outcomes, possibly related to avoiding an increased risk of diabetes.
Lipid management
Atherogenesis is driven by infiltration of low-density lipoprotein-cholesterol (LDL-C) into the arterial wall, meaning LDL-C is a crucial target for prevention of stroke. Lower levels of LDL-C have been consistently associated with a reduction in both MACE and ischaemic stroke risk, but with an increase in haemorrhagic stroke risk via an, as yet, unclear mechanism.15 In patients with ASCVD, ESC guidelines recommend use of lipid-lowering therapy to achieve a LDL-C <1.4 mmol/L. In those without established CV disease, the use of risk scores, such as SCORE2 or QRISK3, can be useful in determining when to offer therapy.14 Statins remain the mainstay of cholesterol-lowering therapy and have proven dose-dependent efficacy in improving net CV outcomes.16 Where LDL-C targets cannot be met with statins, or in cases of intolerance, additional classes of lipid-lowering drug can be useful. Oral options include the selective cholesterol absorption inhibitor ezetimibe and bempedoic acid, the active metabolite of which inhibits adenosine triphosphate-citrate lyase. Parenteral drugs, such as the proprotein convertase subtilisin kexin type (PCSK9) inhibitors alirocumab and evolocumab, and inclisiran, a long-lasting small-interfering ribonucleic acid against the PCSK9 gene, offer particularly impressive reductions in LDL-C.17 A recent development is the discovery of lipoprotein (a) levels as an independent risk factor for CV events, including stroke, and studies are underway to determine if pharmacological modulation of lipoprotein (a) improves clinical outcomes.
Inflammation
Atherogenesis and thrombosis are processes closely linked to inflammation, which is, therefore, another potential target for prevention of stroke. A meta-analysis of 160,309 patients without ASCVD showed a significant association between C-reactive protein (CRP) levels and risk of ischaemic stroke.18 In the CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study) trial, patients with prior MI and high CRP had lower rates of MACE when receiving canakinumab, a monoclonal antibody targeting interleukin-1β, compared with placebo, but with an excess of fatal infections.19 The ability of colchicine to reduce risk of MACE, including stroke, has now been tested in several trials. For example, in the COLCOT (Colchicine Cardiovascular Outcomes Trial) study of patients in the maintenance phase of MI treatment, colchicine impressively and significantly reduced the risk of stroke (a secondary end point) by around 75%, and guidelines acknowledge its consideration to reduce CV risk, though there are still uncertainties given reduction in all-cause mortality has not yet been demonstrated.14 Conversely, methotrexate therapy had no impact on risk of MACE in the CIRT (Cardiovascular Inflammation Reduction Trial) study.2
Optimising glycaemic control in diabetes
Diabetes mellitus is an independent risk factor for cardiovascular diseases including ischaemic and haemorrhagic stroke. In diabetes, each 1% decrease in glycated haemoglobin (HbA1c) towards target is estimated to reduce the relative risk of stroke by 12%.20 Conversely, hypoglycaemia is also associated with increased CV risk, though a specific link to stroke has not been well explored. ESC guidelines recommend a target HbA1c of <7.0% (53 mmol/mol) for reduction in risk of cardiovascular events.14 It is possible that some antidiabetic drug classes play more of a role in stroke prevention than others. For example, in a pooled analysis, glucagon-like peptide-1 (GLP-1) receptor agonists were associated with a significant relative risk reduction of stroke by 13%, whereas sodium-glucose cotransporter-2 (SGLT2) inhibitors were associated with a significant reduction in MACE, but no significant reduction in stroke risk when assessed individually.2
Conclusion
Stroke is a significant cause of mortality and morbidity with a range of risk factors contributing to its pathogenesis. Risk factor assessment and modification form the central basis of stroke prevention. Drugs, alongside lifestyle measures and procedural interventions, can play a key role (figure 2). Stroke risk and prevention should be considered in the context of any concurrent cardiovascular disease or risk factors for adverse effects from therapy, such as bleeding. Ongoing research into stroke prevention strategies will further aid the ability to reduce stroke risk.
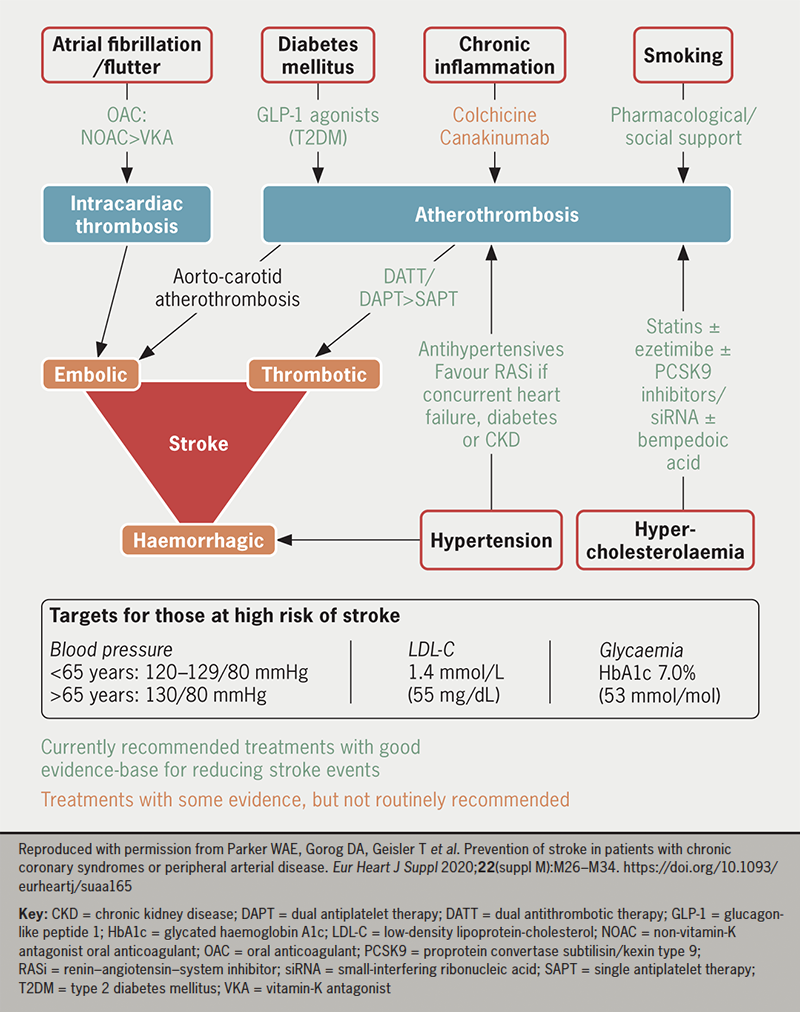
Key messages
- Stroke is responsible for a large burden of mortality and morbidity. Pharmacological strategies to prevent stroke are, therefore, of great importance
- In patients with a history of atrial fibrillation or flutter, oral anticoagulation is recommended in all but the lowest-risk individuals. Antithrombotic therapy can also reduce stroke in patients with established atherosclerotic cardiovascular disease but without atrial tachyarrhythmia
- Good control of blood pressure, lipids, glycaemia and chronic inflammation with appropriate therapies also reduces stroke risk
- When considering whether to initiate drug treatment to prevent stroke, the decision should be made in conjunction with the patient, based on comprehensive assessment of their risk factors
Conflicts of interest
NS: none declared. RFS reports institutional research grants from AstraZeneca, Cytosorbents and GlyCardial Diagnostics; and consultancy fees from AlfaSigma, Alnylam, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb/Pfizer, Chiesi, CSL Behring, Cytosorbents, Daiichi Sankyo, GlyCardial Diagnostics, Hengrui, Idorsia, Novartis, PhaseBio, Sanofi Aventis and Thromboserin. WAEP reports institutional research grants and consultancy fees from AstraZeneca.
Funding
None.
References
1. British Heart Foundation. Heart and circulatory disease statistics 2022. London: BHF, 2022. Available from: https://www.bhf.org.uk/what-we-do/our-research/heart-statistics/heart-statistics-publications/cardiovascular-disease-statistics-2022
2. Parker WAE, Gorog DA, Geisler T et al. Prevention of stroke in patients with chronic coronary syndromes or peripheral arterial disease. Eur Heart J Suppl 2020;22(suppl M):M26–M34. https://doi.org/10.1093/eurheartj/suaa165
3. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. https://doi.org/10.7326/0003-4819-146-12-200706190-00007
4. Hindricks G, Potpara T, Dagres N et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. https://doi.org/10.1093/eurheartj/ehaa612
5. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60. https://doi.org/10.1016/S0140-6736(09)60503-1
6. Parker WAE, Storey RF. Antithrombotic therapy for patients with chronic coronary syndromes. Heart 2021;107:925. https://doi.org/10.1136/heartjnl-2020-316914
7. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348:1329–39. https://doi.org/10.1016/S0140-6736(96)09457-3
8. Hiatt WR, Fowkes FGR, Heizer G et al.; EUCLID Trial Steering Committee and Investigators. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med 2017;376:32–40. https://doi.org/10.1056/NEJMoa1611688
9. Bhatt DL, Fox KAA, Hacke W et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706–17. https://doi.org/10.1056/NEJMoa060989
10. Bonaca MP, Bhatt DL, Cohen M et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–800. https://doi.org/10.1056/NEJMoa1500857
11. Yeh RW, Kereiakes DJ, Steg PG et al. Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol 2015;65:2211–21. https://doi.org/10.1016/j.jacc.2015.03.003
12. Armstrong PW. Extending the product label for ticagrelor: looking under the FDA hood. Circulation 2021;144:583–5. https://doi.org/10.1161/CIRCULATIONAHA.121.055907
13. Eikelboom JW, Connolly SJ, Bosch J et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–30. https://doi.org/10.1056/NEJMoa1709118
14. Visseren FLJ, Mach F, Smulders YM et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice: developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J 2021;42:3227–337. https://doi.org/10.1093/eurheartj/ehab484
15. Sun L, Clarke R, Bennett D et al. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat Med 2019;25:569–74. https://doi.org/10.1038/s41591-019-0366-x
16. Baigent C, Blackwell L, Emberson J et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. https://doi.org/10.1016/S0140-6736(10)61350-5
17. Pierno S, Musumeci O. Pharmacotherapy of the lipid-lowering drugs: update on efficacy and risk. Int J Mol Sci 2023;24:966. https://doi.org/10.3390/ijms24020996
18. Kaptoge S, Di Angelantonio E, Lowe G et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–40. https://doi.org/10.1016/S0140-6736(09)61717-7
19. Ridker PM, Everett BM, Thuren T et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. https://doi.org/10.1056/NEJMoa1707914
20. Stratton IM, Adler AI, Neil HA et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–12. https://doi.org/10.1136/bmj.321.7258.405
