Subclavian venoplasty is commonly performed for subclavian vein stenosis in patients with long-term dialysis lines or fistulae. Such stenosis may also occur in patients with previously implanted intra-cardiac devices. It poses a problem if a further device upgrade or implantation is planned as the stenosis restricts the advancement of leads. Venoplasty before device implantation may provide a feasible alternative to lead tunnelling or extraction, which have their limitations. Four cases of varying complexities and devices that were implanted in patients with subclavian stenosis are presented herein. These were done in a district general hospital within the cardiology team. Venoplasty was performed using peripheral angioplasty balloons after which the device was implanted. All cases were performed successfully without any immediate complications with the patients discharged home the same day. These cases show the utility of subclavian venoplasty in facilitating device implantation without the need to utilise contralateral venous access, hence preserving venous access for the future. Additionally, they illustrate that this may be performed locally in a district general hospital setting, where appropriate expertise is available, with a high success rate and without the need to refer patients to an alternate tertiary care institute which may be associated with additional difficulties for the patient. To the best of our knowledge, this is the first instance where several cases of this procedure were performed successfully in a secondary care setting.
Introduction
Subclavian venoplasty (SV) was developed to re-establish vessel patency following complications from indwelling dialysis catheters.1 Recently, it has been adopted to aid device re-implantation or upgrade, which require the introduction of additional pacing leads; this avoids the need for lead extraction or contralateral tunnelling procedures, both of which are associated with their own technical limitations and procedural complications.2 In suitable patients, SV offers a safer alternative, preserving contralateral access for future use.2 However, these are mostly performed by interventional radiologists rather than cardiologists in tertiary care centres with onsite cardiothoracic surgery capabilities.3,4 Its uptake in secondary care settings is limited. In this series, we present cases where SV was successfully and safely performed to aid complex device implantation in a non-surgical centre setting.
Case presentations
Case 1
A man in his 70s presented to the outpatient cardiology department with increasing breathlessness. He previously underwent pacemaker implantation nine years prior and had a previous history of mild-to-moderate aortic stenosis and Hodgkin’s lymphoma (table 1). His repeat transthoracic echocardiogram showed severely impaired left ventricular systolic function (LVSF) and severe aortic stenosis with a high degree of right ventricular (RV) pacing and non-sustained ventricular tachycardia (VT). His medications included atorvastatin 40 mg once daily (OD), aspirin 75 mg OD, lansoprazole 30 mg OD, levothyroxine 150 µg OD and dapagliflozin 10 mg OD (stopped due to side effects). He was previously on bisoprolol, which was withheld due to a high percentage of RV pacing. He previously had elevated potassium levels (5.5 mmol/L), which prompted the cessation of his renin-angiotensin-aldosterone-system antagonists. In addition to being worked up for aortic valve replacement, it was decided to implant a cardiac resynchronisation therapy-defibrillator (CRT-D) device. A venogram showed an occluded left subclavian vein; therefore, the procedure was stopped at this point and the patient relisted to ensure the required team members and equipment were available. Venoplasty was subsequently successfully performed following which a single-coil active-fixation RV implantable cardioverter-defibrillator (ICD) lead (Sprint Quattro Secure S MRI SureScan, 6935M, Medtronic Ltd, Watford, England) was passed to the RV apex. A coronary sinus (CS) guide catheter was then advanced to the CS ostium; however, it dissected the CS. Therefore, an LV lead implantation was deferred to a later date. A CRT-D generator box (Cobalt HF MRI SureScan, DTPB2QQ, Medtronic Ltd, Watford, England) was connected to the lead and secured in place. An outpatient pacing check showed a reduced degree of RV pacing and therefore, an LV lead was not implanted at this point in time (table 1).
Table 1. Summary of procedures performed
| Case | Previous indication for device implantation | Symptoms | Prior pacing check | LVEF* | Relevant findings on subsequent pacing check |
Device / lead implanted | Immediate complications from SV | Long-term complications | Total follow-up period |
| 1 | Mobitz II second-degree heart block | Breathlessness and pedal oedema | 62% RV pacing with non-sustained VT | 35% | 19% RV pacing | ICD – successful | None. Patient discharged same day | None | 6 months – alive |
| 2 | Third-degree heart block | Breathlessness | 98% RV pacing | 40% | 96% LV pacing | CRT-D – successful | None. Patient discharged same day | None | 21 months – alive |
| 3 | Third-degree heart block | Incidental finding of A-lead malfunction | Non-functioning atrial lead | >55% | Satisfactory checks | Atrial lead – successful | None. Patient discharged same day | None | 18 months – alive |
| 4 | N/A | Syncope and breathlessness. Tachy-brady syndrome on Holter study | N/A | 26% | 93% LV pacing | CRT-P – successful | None. Patient discharged same day | Infected sutures – |
3 months – alive |
| * As determined by transthoracic echocardiography Key: CRT-D = cardiac resynchronisation therapy-defibrillator; CRT-P = cardiac resynchronisation therapy-pacemaker; ICD = implantable cardioverter-defibrillator; LV = left ventricular; LVEF = left ventricular ejection fraction; N/A = not applicable; RV = right ventricular; SV = subclavian venoplasty; VT = ventricular tachycardia |
|||||||||
Case 2
A man in his 70s presented with increasing breathlessness and peripheral oedema. He had a previous history of ischaemic heart disease requiring coronary artery bypass grafting (CABG), atrial fibrillation, a transient ischaemic attack, and complete heart block requiring dual-chamber pacemaker implantation 12 years ago. He was taking edoxaban 60 mg OD, colchicine 500 µg as required, ramipril 5 mg OD, simvastatin 40 mg OD, furosemide 40 mg OD and omeprazole 20 mg OD. An echocardiogram showed a LV ejection fraction of 40% and a pacemaker check showed RV pacing of 98% (table 1). Given that the LV dysfunction was probably secondary to a very high degree of RV pacing i.e. pacemaker-related cardiomyopathy, and less likely to respond to medical therapy alone, he was scheduled for an early upgrade to a CRT-D device in addition to standard medical care. A venogram showed a severe stenosis of the subclavian vein. As this was an unexpected finding and the required equipment for venoplasty was not to hand, the procedure was stopped, and the patient relisted for a staged procedure. This was subsequently successfully performed, after which a single-coil active-fixation RV ICD lead (Reliance 4Front Single CoilDF4, Guidant, 0672, Boston Scientific Corporation, Voisins-le-Bretonneux, France) was advanced to the RV apex. A CS guide catheter (Boston Scientific Corporation, Voisins-le-Bretonneux, France) was then passed to the CS, through which a quadripolar LV lead (Acuity X4 Spiral S, Guidant, 4674, Boston Scientific, Voisins-le-Bretonneux, France) was then passed on to the posterolateral vein with satisfactory parameters. The old generator box was then mobilised and replaced with a new CRT-D device (Resonate X4 CRT-D, Guidant, G447, Boston Scientific, Voisins-le-Bretonneux, France) and secured in place (table 1).
Case 3
A lady in her 60s presented to the pacing clinic for routine follow up. She had a previous history of complete heart block requiring a dual-chamber pacemaker, as well as a history of squamous cell carcinoma and hypothyroidism, for which she was taking levothyroxine 100 µg OD. A routine pacing check showed a non-functioning atrial lead; she was subsequently listed for a redo procedure. A venogram showed subclavian vein occlusion. Since the anatomy appeared amenable to venoplasty, the procedure was stopped at this point and the patient was listed for a later date once all the required equipment and team members with specialist expertise were available. She underwent successful venoplasty, following which a bipolar active-fixation right atrial (RA) lead (Solia S53, 377177, Biotronik UK Ltd, Bicester, UK) was advanced to the atrial appendage with satisfactory parameters. A new generator device was connected to the leads and the previous non-functioning RA lead was then capped and buried (table 1).
Case 4
A lady in her 60s presented with worsening exertional breathlessness. She was previously diagnosed with tachy-brady syndrome, paroxysmal atrial fibrillation and non-Hodgkin’s lymphoma. Her ECG showed a left bundle branch block morphology with a QRS interval longer than 130 ms and severely impaired LVSF. Her medications included candesartan 4 mg OD, dapagliflozin 10 mg OD, spironolactone 12.5 mg OD, bisoprolol 1.25 mg OD, apixaban 5 mg twice daily and hydroxocobalamin as required. She was listed for a cardiac resynchronisation therapy-pacemaker (CRT-P) device. A previous computed tomography (CT) venogram showed severe stenosis of the subclavian vein. Subclavian venoplasty was performed, following which a bipolar active-fixation RV lead (CapSureFix Novus, 5076, Medtronic Ltd, Watford, England) was passed to the RV apex. A bipolar active-fixation RA lead (CapSureFix Novus, 5076, Medtronic Ltd, Watford, England) was then passed to the appendage. A CS guide catheter was then used to engage the coronary sinus and a quadripolar LV lead (Attain Stability Quad 4798, Medtronic Ltd, Watford, England) was then advanced to a suitable-sized vein with satisfactory parameters. A generator box was then connected and secured in place.
Subclavian venoplasty – procedural description
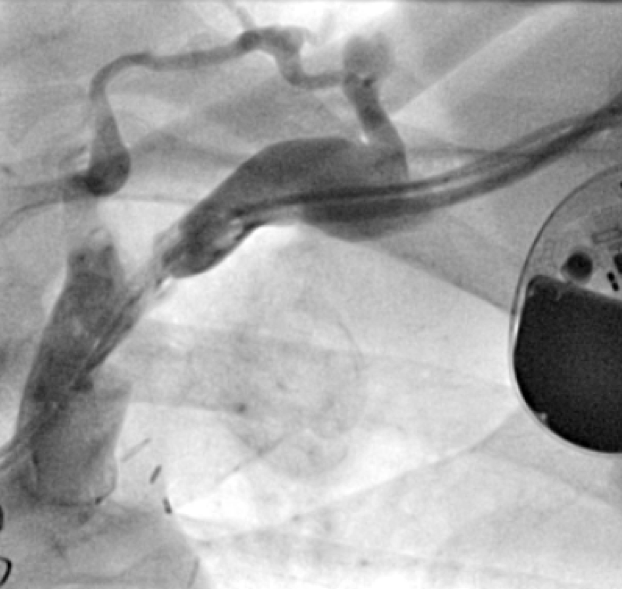
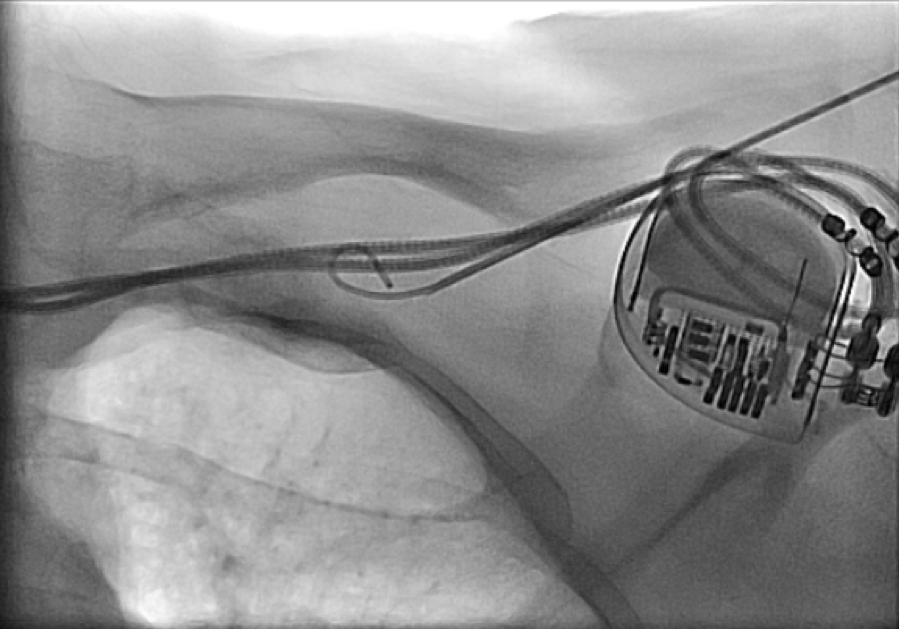
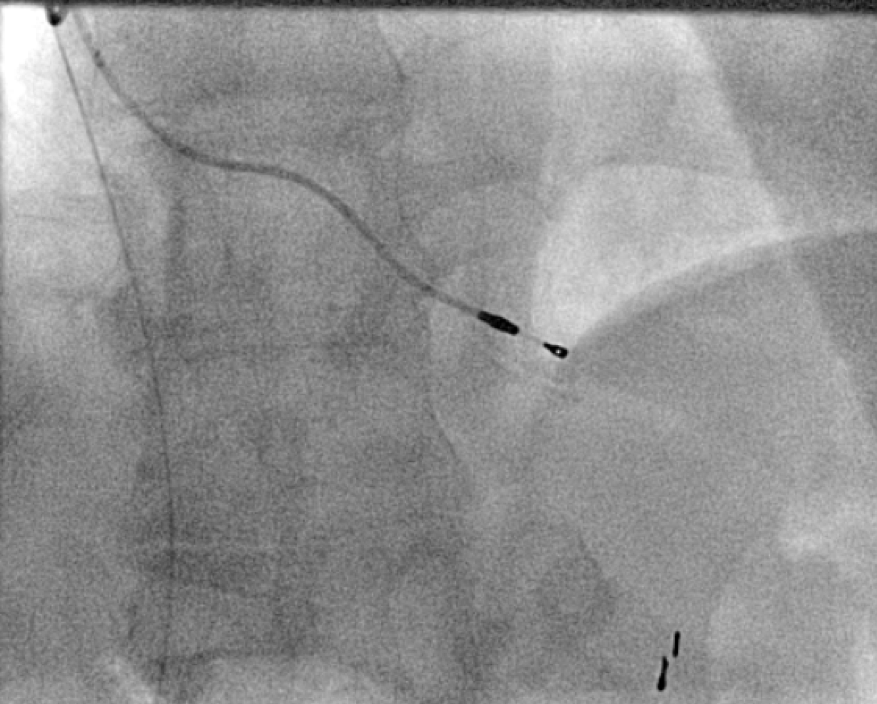
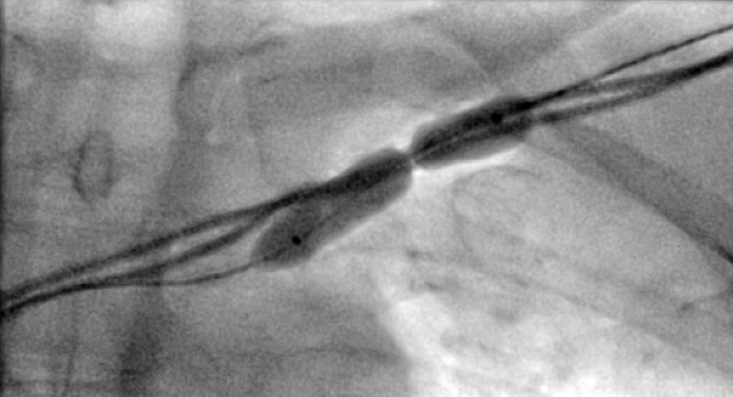
All of the above procedures were performed in our district general hospital. An on-site cardiothoracic surgery service was not available, however, two dedicated tertiary care centres with the required expertise were accessible in the region. Patients were given information beforehand and informed consent was obtained. Complications discussed included those of bleeding, infection, punctured lung, requirement of lung drains and procedure failure. A peripheral venogram was performed using an intravenous line (figure 1). Local anaesthetic (lignocaine 1%) was used to anaesthetise the skin and subcutaneous tissues and then a standard introducer needle was inserted into the vein as far away from the occlusion site as possible, usually over the first or second rib using fluoroscopic guidance, aiming for a shallow angle of 30 degrees (figure 2). The wire was then advanced to the site of the obstruction and a 5-French dilator threaded over the wire. A 0.018-inch nitinol polymer tip hydrophilic wire was then used to cross the occlusion and advanced to the inferior vena cava to ensure that it was not entering into the arterial system. Where the occlusion couldn’t be traversed in this manner (case 1), a Fielder® XT-R (Asahi Intecc Co., Ltd, Aichi, Japan) or HI-TORQUE BALANCE MIDDLEWEIGHT™ (Abbott Vascular Inc, Maidenhead, UK) 0.014-inch guidewire was used. Using a 5-French hydrophilic catheter, the initial guidewire was exchanged for an Amplatz Super Stiff™ (Boston Scientific, Voisins-le-Bretonneux, France) 0.035-inch guidewire (figure 3), ensuring that it tracked into the inferior vena cava to ensure it was in the venous system. Thereafter, a 6 mm × 40 mm-Armada™ (Abbott Vascular Inc, Maidenhead, UK) non-compliant balloon was then sited just beyond the superior vena cava and innominate junction. An 8 or 9 mm Armada (Abbott Vascular Inc, Maidenhead, UK) balloon was used if more than one lead was planned to be introduced or if there was elastic recoil after the 6-mm balloon inflations (figures 4 & 5). Shorter balloons, such as 20-mm noncompliant balloons or even coronary angioplasty balloons, were needed at times when the initial balloons failed across the occlusion. The balloons were inflated at nominal pressures and care was taken to ensure that the balloons were inflated for long enough that there was no residual waisting noted. The balloon was then deflated and retracted until visualised within the pocket. A 7-French sheath was then advanced on the wire, following which the device leads were introduced and secured in place, as per routine practice (figure 6). Post-procedure postero-anterior and lateral chest X-rays and pacing checks were performed on all patients.
Discussion
Up to 50% of patients with implanted cardiac devices may develop subclavian vein stenosis; the majority of which remain asymptomatic due to development of collateral venous supply.5 Underlying mechanisms comprise early or delayed venous thrombosis and fibrotic hyperplasia, primarily from endothelial injury at the site of repeated mechanical trauma.1,5 SV has been around for almost 40 years.1 It was developed to relieve obstruction in symptomatic patients as an alternative to surgical resection in the context of patients with haemodialysis lines,1,6 although other causes such as pacemaker leads, radiation trauma and external compression would also lead to similar issues.1,5,6 More recently, it has been adopted to facilitate cardiac device implantation or upgrade.
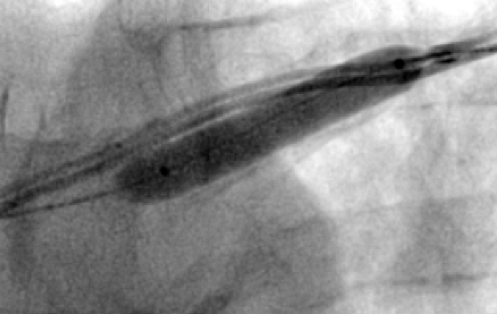 |
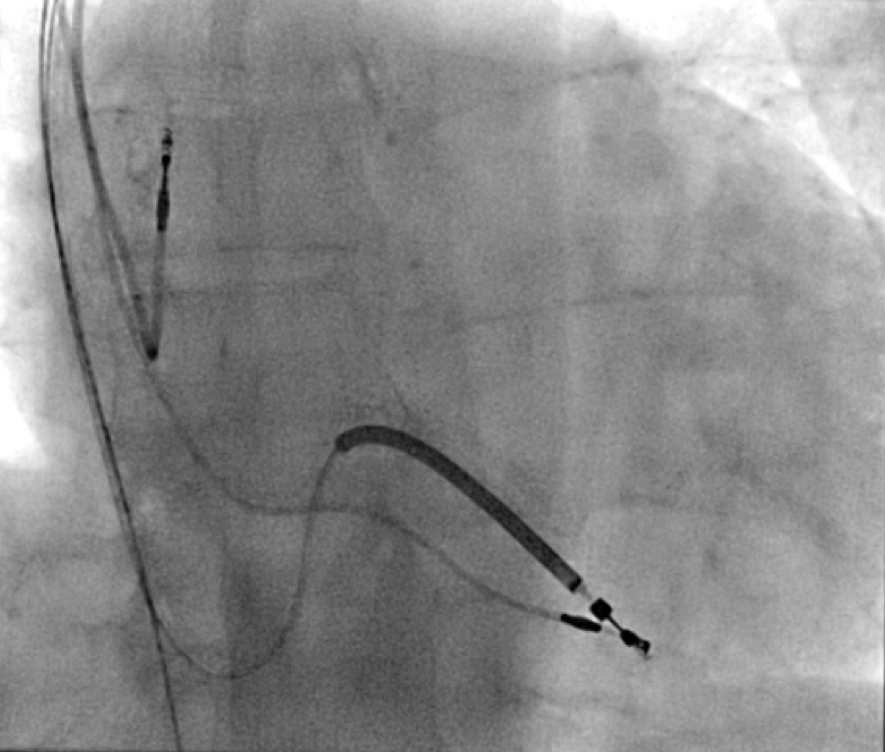 |
Kim et al. performed successful SV and device implantation in a patient with symptomatic complete heart block who had developed stenosis from previous device implantation.7 Other case reports suggest similar utility and feasibility of the procedure in aiding device implantation or revision.8 Worley et al. showed that of 373 patients with subclavian venous stenosis who either required device implantation or upgrade, 371 patients successfully underwent the procedure with SV.3 Of the patients who were followed up, no complications were reported and existing leads remained intact.3 An extended review by the same group showed that two atrial lead dislodgements and one lead insulation defect was found following the procedure when performed in 488 patients.5 SV may be quicker in comparison to progressive catheter dilation, which may not only be higher risk but may also limit catheter manipulation making lead placements difficult.5 Another review of 84 patients by Maass and colleagues in two Dutch tertiary care centres reported a success rate of 91% with 4% patients developing pocket hematomas, managed conservatively, and 1% requiring lead reposition due to dislodgement.3 There was no damage detected to any existing leads and 89% had good recovery without any complications.3 The 2017 Heart Rhythm Society expert consensus also highlights these aspects with regards to SV, recommending it as one of the methods of addressing the issue of subclavian vein stenosis, with the other options including contralateral venous access, tunnelled line and lead extraction.2 The latter in particular, can be high risk and requires the availability of onsite surgical backup in case any serious complications arise.2 Similarly, contralateral venous access and tunnelling result in the utilisation of additional venous access points and, therefore, limit further access options for the future,2 which may be especially important in younger patients. Moreover, complications may be associated with this approach including ventricular over-sensing (5.5%), local wound complications (11.1%) which may require extraction (5.5%), and discomfort (5.5%), as reported in one series.9 Therefore, in appropriate patients, SV may be safer and quicker with a high success rate.
The procedures performed in these cases above were all carried out in tertiary care centres. Others recommend they be performed by electrophysiologists;10 such expertise is usually available only in tertiary care centres. These procedures were performed in a district general hospital with no onsite cardiothoracic surgery cover or electrophysiologists. However, there was access to the cardiothoracic teams at two local tertiary care centres (separate from the hospital) in case required. These links were previously developed as part of the site’s primary percutaneous coronary intervention service for the management of patients with ST-elevation myocardial infarction. SV was performed successfully by the existing cardiology team comprising a cardiac device-implanting physician and an interventional cardiologist with the appropriate expertise in peripheral angioplasty. With no immediate complications, these cases highlight the feasibility and safety of performing such procedures in a district general hospital setting in the UK without the need to refer to a tertiary care centre or for more complex procedures such as device extraction. To the best of our knowledge, we report the first instance in which a series of cases have been performed successfully in this manner in our region. Additionally, this series highlights the efficacy, safety and success rate of SV in a variety of clinical scenarios, ranging from implantation of single atrial pacing leads to implantation of more complex devices such as ICDs and CRTs. Furthermore, these patients constituted individuals with de novo subclavian vein stenosis, as well as those with previous device, leads and CABG in whom tunnelling would have been proven to be difficult – SV provided a safe alternative in these patients. Similarly, CT venography, if performed previously, may be able to detect subclavian venous stenosis early on and help in adequate procedure planning and the avoidance of multiple admissions. However, whilst studies have looked at CT-guided LV lead implantation to improve results,11,12 routine CT venography prior to device upgrade is currently limited by service availability and may result in unnecessary delays in device implantation. Therefore, it should only be performed where there is a high index of suspicion of subclavian vein stenosis.
In summary, this case series demonstrates that SV can be used successfully to allow device implantation with good clinical outcomes in a secondary care setting. Centres with the available expertise should consider the performance of SV locally to allow timely management of patients and reduce the burden on tertiary care centres.
Key messages
- Subclavian vein stenosis may be frequently encountered in patients with previously implanted cardiac devices or those previously treated with radiotherapy
- Subclavian venoplasty with follow-on device implantation presents a feasible option in suitable patients with subclavian vein stenosis
- In secondary care centres with suitable expertise, this procedure may be safely performed and allow early management of patients and the avoidance of referral to tertiary care centres which may delay further care and also avoid the need to travel to other centres.
Conflicts of interest
None declared.
Funding
None.
Statement of consent
Informed consent to publish was obtained from all patients discussed in this case series.
References
1. Al Jamil A. Venoplasty: a less frequent but essential procedure. Scientific Track Abstracts at International Surgery and Ortho Conference, Toronto, Canada, October 25–26, 2017. Available at: https://www.alliedacademies.org/proceedings/venoplasty-a-less-frequent-but-essential-procedure-571.html (accessed July 2024)
2. Kusumoto FM, Schoenfeld MH, Wilkoff BL et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017;14:e503–51. https://doi.org/10.1016/j.hrthm.2017.09.001 [Epub online ahead of print]
3. Worley SJ, Gohn DC, Pulliam RW, Raifsnider MA, Ebersole BI, Tuzi J. Subclavian venoplasty by the implanting physicians in 373 patients over 11 years. Heart Rhythm 2011;8:526–33. https://doi.org/10.1016/j.hrthm.2010.12.014 [Epub online ahead of print]
4. Maass AH, Lankveld T, Groenveld HF et al. Venoplasty can be performed safely and successfully in patients with subtotal and total venous occlusion needing additional transvenous electrodes. Heart Rhythm 2023;20(Suppl):S380–S381. https://doi.org/10.1016/j.hrthm.2023.03.868
5. Marcial JM, Worley SJ. Venous system interventions for device implantation. Card Electrophysiol Clin 2018;10:163–77. https://doi.org/10.1016/j.ccep.2017.11.017
6. Das D, Acharya D, Das T, Singh J, Pramanik S. Subclavian venoplasty: a second life to the upper limb. Indian J Case Reports 2021;7:191–3. https://doi.org/10.32677/IJCR.2021.v07.i05.006
7. Kim JS, Pak H-N, Lim HE, Kim Y-H. A case of pacemaker implantation after balloon venoplasty on innominate vein stenosis. Korean Circ J 2005;35:558–61.
8. Moukabary T, Thai H, Thal S. Subclavian balloon venoplasty to facilitate lead implant in patient with subclavian venous obstruction. J Invasive Cardiol 2011;23:e83–e85.
9. Lüthje L, Zabel M, Seegers J, Zenker D, Vollmann D. Acute and long-term feasibility of contralateral transvenous lead placement with subcutaneous, pre-sternal tunnelling in patients with chronically implanted rhythm devices. Europace 2011;13:1004–8. https://doi.org/10.1093/europace/eur072 [Epub online ahead of print]
10. Ji SY, Gundewar S, Palma EC. Subclavian venoplasty may reduce implant times and implant failures in the era of increasing device upgrades. Pacing Clin Electrophysiol 2012;35:444–8. https://doi.org/10.1111/j.1540-8159.2011.03303.x [Epub online ahead of print]
11. Gould J, Sidhu BS, Sieniewicz BJ et al. Feasibility of intraprocedural integration of cardiac CT to guide left ventricular lead implantation for CRT upgrades. J Cardiovasc Electrophysiol 2021;32:802–12. https://doi.org/10.1111/jce.14896 [Epub online ahead of print]
12. Behar JM, Rajani R, Pourmorteza A et al. Comprehensive use of cardiac computed tomography to guide left ventricular lead placement in cardiac resynchronization therapy. Heart Rhythm 2017;14:1364–72. https://doi.org/10.1016/j.hrthm.2017.04.041 [Epub online ahead of print]
