The purpose of this review is to give a broad overview of the role of patent foramen ovale (PFO) in disease. The embryological origins of PFO are described before reviewing the different diagnostic modalities available, including transthoracic echocardiography, trans-oesophageal echocardiography and transcranial ultrasound scanning. The role, or proposed role, of PFO in conditions including cryptogenic stroke, decompression sickness and migraine are discussed, as well as different treatment options, including the evidence for closure of PFO. Some of the range of methods and devices used to close PFO are described, as are the possible complications when attempting closure.
Introduction
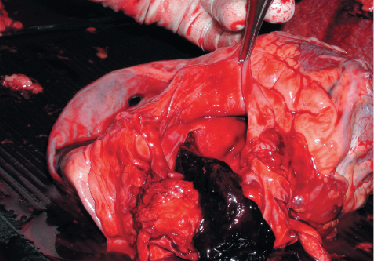
The first case of pathology resulting from a patent foramen ovale (PFO) is thought to have been described by Julius Cohnheim,1 who, in 1877, presented the case of a 35-year-old woman who died after a paradoxical embolus had led to fatal cerebral embolism. With time, more pathologies have become associated with, and been attributed to, PFO (figure 1). In this article, we review the diagnosis of PFO and indications for its closure, as well as the methods and complications of closure.
Embryology of PFO
The primitive embryonic atrium starts as a single cavity. The septum primum grows caudally from the top of the atrium down towards the endocardial cushion. The area between the leading edge of the septum primum and the endocardial cushion is known as the ostium primum, which closes when the septum primum fuses with the endocardial cushion. A second orifice, the ostium secundum, develops at the top of the septum primum following the coalescence of multiple small perforations. The septum secundum then develops on the right atrial side of the septum primum. The septum secundum covers the ostium secundum but does not completely divide the atria. This persisting orifice within the septum secundum is the foramen ovale. The foramen ovale allows the shunting of oxygenated blood from the right atrium into the left atrium and, thereafter, into the systemic circulation. Following birth, the changes in pressure within the atria lead to closure of the foramen ovale in the majority. A PFO results from a failure to close and persists in around 25% of adults.2
Diagnosis of PFO
In vivo diagnosis of PFO was difficult until the advent of ultrasound. A variety of ultrasound modalities, including transthoracic echocardiography (TTE), trans-oesophageal echocardiography (TOE) and transcranial Doppler (TCD) are now available to aid diagnosis (figure 2).
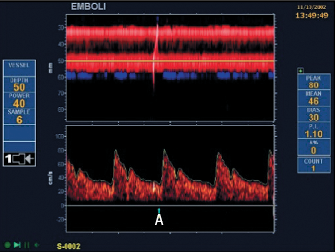
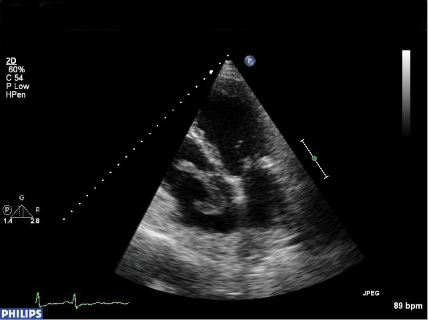
All rely on the injection of ‘bubble contrast’ (a mixture of saline, air and blood) into a peripheral vein combined with procedures to increase pressure in the right atrium (Valsalva release, sniff, etc.). With TOE and TTE, visualisation of movement of bubbles from the right atrium (RA) into the left atrium (LA) within three to five cardiac cycles demonstrates the presence of a right-to-left intracardiac shunt. TCD demonstrates right to left shunting (not necessarily intracardiac) by detecting the presence of contrast in the middle cerebral artery as ‘hits’. TOE has previously been considered the ‘gold standard’ investigation as it has a high sensitivity and specificity in the diagnosis of PFO.3,4 It is, however, an invasive investigation that requires sedation and causes discomfort to the patient, and limits the patient’s ability to perform an adequate Valsalva. TCD is a non-invasive and sensitive investigation with a high sensitivity in the detection of PFO,5 but cannot differentiate an intracardiac from a pulmonary shunt. TTE is also non-invasive and, with the development of second harmonic imaging, studies have suggested that it may have a sensitivity equivalent to TOE in the detection of PFO and is more cost-effective.6,7 It is for this reason that TTE with bubble contrast has become the screening investigation of choice for a PFO at most centres.
Indications for closure
Paradoxical embolisation
A PFO can allow paradoxical embolisation of thrombus, or any other circulating material, from the right-sided venous circulation into the left-sided arterial circulation. This can result in damage to the brain, eyes, heart, kidneys or other organs (figure 3). The most sensitive organ to this type of embolisation is the brain. A commonly used classification system for stroke was first devised for research purposes before being modified for clinical use in the Trial of Org 10172 in Acute Stroke Treatment (TOAST).8 This defined a cryptogenic stroke as one where there is ischaemic stroke not attributable to a source of definite cardioembolism, large artery atherosclerosis or small vessel disease, despite thorough investigation. A meta-analysis of case–control studies confirmed a high incidence of PFO in patients under the age of 55 with cryptogenic stroke.9 In older patients, the association is not as clear, with several studies showing no association,10,11 while a more recent study did show a positive association.12
The presence of a PFO increases the risk of recurrent stroke.9 Treatment modalities for secondary prevention of stroke in patients with a PFO include medical treatment (warfarin or antiplatelet agents) or closure of the PFO. A review of non-randomised trials suggests that the rate of recurrence of stroke is at least as low or lower with PFO closure compared with medical treatment.13,14 However, preliminary results reported at the American Heart Association meeting in 2010 from the CLOSURE I trial, which randomised 900 patients to either medical therapy or transcatheter closure of PFO, showed no benefit in PFO closure over best medical management in preventing recurrence of stroke or transient ischaemic attack.
When evaluating a patient with a PFO following a cryptogenic stroke, there can be some uncertainty as to whether the PFO is directly responsible for the stroke. Alsheikh-Ali et al. made an attempt to quantify the probability that the finding of a PFO in a patient with an otherwise cryptogenic stroke was incidental.15 They carried out an analysis of a series of case–control studies looking at the prevalence of PFO in patients with cryptogenic stroke compared with patients with stroke of known origin. This analysis (using mathematical modelling and some simple assumptions) suggested that the presence of a PFO in a patient with a cryptogenic stroke may be incidental in up to 33% of cases (this figure is lower in young patients and higher in older patients).
Decompression sickness in divers
Decompression sickness (‘the bends’) occurs when bubbles form in the tissues as a result of a change in pressure causing dissolved gases (principally nitrogen) to enter a gaseous state. This can affect those ascending from depth (e.g. divers), those ascending to altitude and those leaving a high-pressure environment. In divers, the risk of decompression sickness is increased by the duration and depth of dives, especially if ascent is carried out without acclimatisation. PFOs have been associated with neurological decompression sickness in divers.16,17 Hyperintense lesions in the brain and spinal cord of divers have been demonstrated on magnetic resonance imaging (MRI), even in the absence of decompression sickness.18 The number of these lesions is significantly greater in divers with a PFO compared with those without.19 There is evidence that divers with a PFO who have suffered decompression sickness after a theoretically safe dive profile benefit from its closure.20,21
Platypnoea orthodeoxia
Platypnoea-orthodeoxia syndrome (POS) is a rare condition characterised by dyspnoea and arterial desaturation in the upright position that is relieved in the recumbent position. It is associated with post-pneumonectomy state, aortic aneurysm and cirrhosis of the liver, in the presence of right-to-left intracardiac shunting, usually due to PFO.22 Although the mechanism by which shunting is present in the upright position, but disappears in the recumbent position, has not been fully elucidated, there is evidence that closure of the intracardiac shunt relieves symptoms.23 In the past, the only treatment option available has been open heart surgery, however, case series in patients with POS have shown that transcatheter closure of PFO with an occluder device is safe and results in marked improvement in dyspnoea and desaturation.24
Migraine with aura
Migraine is a common condition that affects up to 13% of the population aged between 18 and 55 years.25 Interest in the role of PFO in migraine first arose when it was shown that the prevalence of PFO was higher in patients who suffered migraine with aura compared with healthy controls (41% in migraine with aura versus 16% in controls).26 Wilmshurst et al. carried out a retrospective analysis of patients who had their PFO closed for other indications (decompression illness, cryptogenic stroke) and showed that there had been a significant improvement in migraine symptoms.27 A subsequent small prospective study where PFOs were closed following cryptogenic stroke confirmed these findings.28 One proposed mechanism of causation is that venous blood contains unknown agents, normally filtered by the lungs, which enter the cerebral circulation to trigger migraine in those with a right-to-left shunt. There was, therefore, much interest in the Migraine Intervention with STAR Flex Technology Trial (MIST),29 a prospective, double-blind, randomised trial assessing the effectiveness of PFO closure in the treatment of migraine. Closure of PFO did not result in cure for migraine, although post hoc analyses with exclusion of statistical outliers suggested that the number of ‘migraine days’ was significantly reduced. There is ongoing controversy around this trial with one of the original trial investigators currently involved in a libel case with a device manufacturer.30 At present, there is not enough evidence to advocate closure of PFO in migraine unless it is part of a clinical trial.
Transcatheter closure of PFO
Transcatheter closure of a PFO to treat presumed paradoxical embolisation was first described by Bridges et al.,31 although closure of an atrial septal defect in congenital heart disease had been performed during cardiac catheterisation almost two decades previously.32 This procedure is now commonly performed around the world for the indications already described. There is wide variation both in the imaging used during closure and in the choice of closure device. Most commonly, imaging during closure involves intra-procedural TOE with the patient either anaesthetised or heavily sedated. Another option is intracardiac echocardiography (ICE) during the procedure. This is a technique that uses ultrasound tipped cardiac catheters that give good views of a PFO during attempted closure.33 The main disadvantages of this system are the high costs currently involved and the increased risk of access site complications. Other groups have advocated closure using fluoroscopy alone, with TOE having been carried out pre-procedure to confirm the presence of a PFO and to delineate anatomy.34,35 The main advantage of this is that the patient does not need to be sedated or anaesthetised and, therefore, time and cost benefits arise. This technique requires a greater degree of technical expertise and may not be available at all centres.
At the beginning of the closure procedure, a catheter is inserted at the right femoral vein to enable access to the right atrium. A balloon is then used to size the PFO, giving information both on the size and tunnel length. This, as well as the pre-procedure TOE provides vital information to guide the choice of closure device to be used. In the presence of a thick secondary septum, an articulating device that conforms to the septum may give better closure than a non-articulating device, which will sit off the septum on its right atrial aspect. Such devices include Starflex and Biostar (NMT Medical) (figures 4 and 5). These devices consist of a double umbrella with either Dacron (Starflex) or porcine collagen patches (Biostar) on an alloy framework with a self-centring mechanism consisting of micro-springs. If the tunnel length is long, either a strong device may be chosen, which can effectively concertina the interatrial septal tunnel to close the PFO, such as the Amplatzer PFO Occluder device (AGA Medical Corporation), or a device designed to sit in the tunnel and obliterate it (FlatStent from Coherex).

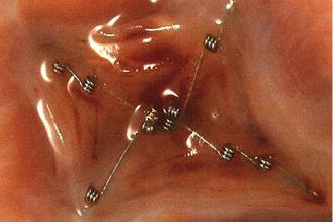
Alternatively, the operator may choose to close the PFO using a trans-septal puncture at the base of the secondary septum. A number of devices may be suitable in the latter. The size and type will be determined by the integrity (or sturdiness) of the atrial septum and the area needed to ensure the defect is fully covered. A wide-based tunnel usually occurs with large PFOs and may have implications for planning (e.g. planned intracardiac echocardiography). The Premere PFO Closure System (St Jude Medical) is the only variable waist device and, in theory, is well suited to closure of a long tunnel PFO. It consists of two nitinol atrial anchors (left and right) that are attached by a polyester thread. The thread can be locked at a length that is suitable for the PFO tunnel. An atrial septal aneurysm is defined as redundant or excessive tissue of the atrial septum that may sway into either atrium with a total excursion from the midline of >15 mm, or in one direction >10 mm. Atrial septal aneurysms are often associated with larger PFOs. Some operators plan to cover a large area of the septum in the presence of an aneurysm, effectively ‘stapling’ the septum (e.g. Amplatzer PFO and Cribriform Occluder devices), while others prefer to use a soft and malleable device (such as the HELEX septal occluder [W.L. Gore & Medical Associates], or the Starflex device) and allow it to conform to the existing anatomy.
After selection of the device, a wire is used to cross the PFO and access the left atrium before the closure device is deployed across it. The specifics of the deployment vary according to the device used. Antiplatelet agents are used for three to six months following the procedure to reduce the risks of clot formation on the device. The effectiveness of the closure can be checked with bubble contrast TTE at six months to examine for any residual shunt.
Complications of percutaneous closure of PFO
Closure of a PFO remains an invasive procedure and is not without complications, though as heart procedures go, it is one of the safest. One review of 1,970 patients in 11 different studies who underwent percutaneous PFO closure found cardiac tamponade in 0.3%, transient ischaemic attacks (TIAs) in 0.2%, device embolisation (and successful retrieval) in 1.1% and vascular access site problems in 1.5%.13 There were no reported deaths, myocardial infarctions or stroke. Another review of 1,349 patients found new onset of atrial fibrillation (AF) within four weeks of closure, with the arrhythmia being persistent in around half these cases.36 However, a comparison of the rate of new onset AF between patients undergoing PFO closure and patients with stroke due to a different cause found no significant differences.37 As cardiologists become more experienced with the implantation of PFO closure devices, it is likely that the rates of complications will lessen.
Conclusions
PFO closure is a safe procedure with a low number of reported serious complications. Patients with cryptogenic stroke or other systemic embolisation should be screened for PFO and closure offered. Patients with decompression sickness who wish to continue to dive should have their PFO closed, as should patients with platypnoea orthodeoxia.
Conflict of interest
SG: none declared. DHS has consulted for AGA medical, Coherex and Gore.
Key messages
- Patent foramen ovale (PFO) are common, occurring in around 25% of the population
- PFO have been implicated in a range of medical problems
- There is ongoing debate about the best management of PFO
- In carefully selected patients, PFO closure is a safe and viable alternative to medical management when PFO have caused disease
References
- Lippmann H, Rafferty T. Patent foramen ovale and paradoxical embolization: a historical perspective. Yale J Biol Med 1993;66:11–17.
- Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984;59:17–20.
- Pinto FJ. When and how to diagnose patent foramen ovale. Heart 2005;91:438–40. (doi: 10.1136/hrt.2004.052233)
- Di Tullio M, Sacco RL, Venketasubramanian N, Sherman D, Mohr JP, Homma S. Comparison of diagnostic techniques for the detection of a patent foramen ovale in stroke patients. Stroke 1993;24:1020–4. (doi: 10.1161/01.STR.24.7.1020)
- Teague SM, Sharma MK. Detection of paradoxical cerebral echo contrast embolization by transcranial Doppler ultrasound. Stroke 1991;22:740–5. (doi: 10.1161/01.STR.22.6.740)
- Daniëls C, Weytjens C, Cosyns B et al. Second harmonic transthoracic echocardiography: the new reference screening method for the detection of patent foramen ovale. Eur J Echocardiogr 2004;5:449–52. (doi: 10.1016/j.euje.2004.04.004)
- Thanigaraj S, Valika A, Zajarias A, Lasala JM, Perez JE. Comparison of transthoracic versus transesophageal echocardiography for detection of right-to-left atrial shunting using agitated saline contrast. Am J Cardiol 2005;96:1007–10. (doi: 10.1016/j.amjcard.2005.05.061)
- Adams HP, Bendixen BH, Kappelle LJ et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. (doi: 10.1161/01.STR.24.1.35)
- Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology 2000;55:1172–9.
- Hausmann D, Mügge A, Becht I, Daniel WG. Diagnosis of patent foramen ovale by transesophageal echocardiography and association with cerebral and peripheral embolic events. Am J Cardiol 1992;70:668–72. (doi: 10.1016/0002-9149(92)90210-P)
- Jones EF, Calafiore P, Donnan GA, Tonkin AM. Evidence that patent foramen ovale is not a risk factor for cerebral ischemia in the elderly. Am J Cardiol 1994;74:596–9. (doi: 10.1016/0002-9149(94)90750-1)
- Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med 2007;357:2262–8. (doi: 10.1056/NEJMoa071422)
- Wöhrle J. Closure of patent foramen ovale after cryptogenic stroke. Lancet 2006;368:350–2. (doi: 10.1016/S0140-6736(06)69087-9)
- Windecker S, Wahl A, Nedeltchev K et al. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J Am Coll Cardiol 2004;44:750–8. (doi: 10.1016/j.jacc.2004.05.044)
- Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke 2009;40:2349–55. (doi: 10.1161/STROKEAHA.109.547828)
- Moon R, Camporesi E, Kisslo J. Patent foramen ovale and decompression sickness in divers. Lancet 1989;333:513–14. (doi: 10.1016/S0140-6736(89)90064-0)
- Wilmshurst PT, Byrne JC, Webb-Peploe MM. Relation between interatrial shunts and decompression sickness in divers. Lancet 1989;2:1302–06. (doi: 10.1016/S0140-6736(89)91911-9)
- Reul J, Weis J, Jung A, Willmes K, Thron A. Central nervous system lesions and cervical disc herniations in amateur divers. Lancet 1995;345:1403–05. (doi: 10.1016/50140-6736(95)92598-8)
- Knauth M, Ries S, Pohimann S et al. Cohort study of multiple brain lesions in sport divers: role of a patent foramen ovale. BMJ 1997;314:701.
- Wilmshurst P, Walsh K, Morrison L. Transcatheter occlusion of foramen ovale with a button device after neurological decompression illness in professional divers. Lancet 1996;348:752–3. (doi: 10.1016/S0140-6736(05)65638-3)
- Walsh KP, Wilmshurst PT, Morrison WL. Transcatheter closure of patent foramen ovale using the Amplatzer septal occluder to prevent recurrence of neurological decompression illness in divers. Heart 1999;81:257–61.
- Faller M, Kessler R, Chaouat A, Ehrhart M, Petit H, Weitzenblum E. Platypnea-orthodeoxia syndrome related to an aortic aneurysm combined with an aneurysm of the atrial septum. Chest 2000;118:553–7. (doi: 10.1378/chest.118.2.553)
- Acharya SS, Kartan R. A case of orthodeoxia caused by an atrial septal aneurysm. Chest 2000;118:871–4. (doi: 10.1378/chest.118.3.871)
- Rao PS, Palacios IF, Bach RG, Bitar SR, Sideris EB. Platypnea-orthodeoxia: management by transcatheter buttoned device implantation. Catheter Cardiovasc Interv 2001;54:77–82. (doi: 10.1002/ccd.1243)
- Lipton RB, Liberman JN, Kolodner KB, Bigal ME, Dowson A, Stewart WF. Migraine headache disability and health-related quality-of-life: a population-based case-control study from England. Cephalalgia 2003;23:441–50. (doi: 10.1046/j.1468-2982.2003.00546.x)
- Anzola GP, Magoni M, Guindani M, Rozzini L, Dalla Volta G. Potential source of cerebral embolism in migraine with aura: a transcranial Doppler study. Neurology 1999;52:1622–5.
- Wilmshurst PT, Nightingale S, Walsh KP, Morrison WL. Effect on migraine of closure of cardiac right-to-left shunts to prevent recurrence of decompression illness or stroke or for haemodynamic reasons. Lancet 2000;356:1648–51. (doi: 10.1016/S0140-6736(00)03160-3)
- Morandi E, Anzola GP, Angeli S, Melzi G, Onorato E. Transcatheter closure of patent foramen ovale: a new migraine treatment? J Interv Cardiol 2003;16:39–42. (doi: 10.1046/j.1540-8183.2003.08001.x)
- Dowson A, Mullen MJ, Peatfield R et al. Migraine Intervention With STARFlex Technology (MIST) trial: a prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation 2008;117:1397–404. (doi: 10.1161/CIRCULATIONAHA.107.727271)
- Gornall J. A very public break-up. BMJ 2010;340:c110. (doi: 10.1136/bmj.c110)
- Bridges ND, Hellenbrand W, Latson L, Filiano J, Newburger JW, Lock JE. Transcatheter closure of patent foramen ovale after presumed paradoxical embolism. Circulation 1992;86:1902–08.
- King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA 1976;235:2506–09. (doi: 10.1001/jama.235.23.2506)
- Koenig P, Cao Q, Heitschmidt M, Waight DJ, Hijazi ZM. Role of intracardiac echocardiographic guidance in transcatheter closure of atrial septal defects and patent foramen ovale using the Amplatzer device. J Interv Cardiol 2003;16:51–62. (doi: 10.1046/j.1540-8183.2003.08003.x)
- Wahl A, Praz F, Stinimann J et al. Safety and feasibility of percutaneous closure of patent foramen ovale without intra-procedural echocardiography in 825 patients. Swiss Med Wkly 2008;138:567–72.
- Hildick-Smith D, Behan M, Haworth P, Rana B, Thomas M. Patent foramen ovale closure without echocardiographic control: use of “standby” intracardiac ultrasound. JACC Cardiovasc Interv 2008;1:387–91. (doi: 10.1016/j.jcin.2008.05.006)
- Staubach S, Steinberg DH, Zimmermann W et al. New onset atrial fibrillation after patent foramen ovale closure. Catheter Cardiovasc Interv 2009;74:889–95. (doi: 10.1002/ccd.22172)
- Burow A, Schwerzmann M, Wallmann D et al. Atrial fibrillation following device closure of patent foramen ovale. Cardiology 2008;111:47–50. (doi: 10.1159/000113427)
