The diagnosis and management of hypertrophic cardiomyopathy (HCM) has undergone fundamental change since the condition was first described more than 50 years ago by Donald Teare,1 a forensic pathologist, and Michael Davis, an academic pathologist.
HCM is conventionally understood as a cardiac disease inherited in an autosomal dominant fashion with incomplete penetrance. Over the past two decades, genetic research has established that HCM has considerable allelic and non-allelic heterogeneity. For the majority of patients, outside of its utility in pre-symptomatic screening, a genetic diagnosis has not made important contributions to clinical management. In large part, this is because most proband patients have apparently unique mutations, and because the fidelity between genotype and phenotype has been poor in families and for mutations studied thus far.
Over the last 20 years, the diagnosis of HCM has been driven primarily by echocardiography – the key imaging modality for establishing the diagnosis of HCM in routine cardiological practice. More recently, however, increasing access to cardiac magnetic resonance imaging (MRI) has led to an increased number of patients with a secure diagnosis of HCM, requiring counselling and treatment. In our centre, which performs over 3,000 cardiac MRI scans per year, over 200 (~7%) patients are given a new diagnosis of unexplained left ventricular hypertrophy per year. This improvement in non-invasive imaging modalities will also increase the number of patients with HCM who have significant co-morbidities, making them less attractive for surgical treatment. These factors inevitably mean that interest in non-surgical treatments for patients with HCM, such as alcohol septal ablation (ASA), will continue to increase.
The paper by Hamid et al. (see pages 233–7), provides a useful analysis of the current clinical role of ASA in the management of symptomatic patients with obstructive HCM.
Patient selection
Hamid et al. correctly emphasise that intervention with ASA should be restricted to those patients whose symptoms of breathlessness or chest pain suggestive of myocardial ischaemia (New York Heart Association [NYHA] class III or IV) are resistant to medical therapy. As stressed in the American College of Cardiology/European Society of Cardiology (ACC/ESC) guidelines,2 the sub-aortic gradient should be 50 mmHg or more as assessed by Doppler echocardiography, either under basal resting conditions or under physiological stress, such as Valsalva.
The anatomical requirement for ASA is for a first septal perforator of sufficiently large caliber to allow an over-the-wire balloon to be inflated to occlude the vessel (usually ≥1.5 mm). This vessel must supply an appropriate area of the basal septum assessed by contrast echocardiography. The mechanism of action is that the alcohol diffuses through the myocardium causing a direct chemical permanent necrosis of the myocytes, rather than just transient myocardial ischaemia caused by occlusion of the (usually well-collateralised) septal artery. The size of the iatrogenic infarct can be controlled relatively precisely and usually, the appropriate septal perforator remains patent for further procedures if required.
ASA has an impressive effect in improving symptoms.2,3 In their study, Hamid et al. reported an improvement from NYHA III/IV to NYHA 0–II in 87% of their cohort at three months and 67% at nine months.It is, however, important to note, that although studies have shown that HCM patients with obstruction have worse overall survival and HCM-related survival than HCM patients without obstruction,4,5 there is no evidence to date that either myectomy (Morrow resection) or ASA improve prognosis.
Hamid et al. have emphasised that the maximal physiological reduction in the outflow gradient occurs between six and 12 months, and this has also been our experience, although the amount of ethanol used per case by us was much less (mean 1.6 ± 0.57 ml) compared with that in the study group (4.4 ± 2 ml). In our series of 56 consecutive patients with obstructive HCM who underwent ASA, 14% (eight patients) developed complete heart block post-procedure requiring permanent pacemaker implantation compared with 20% (three patients) in the study group.
Future development
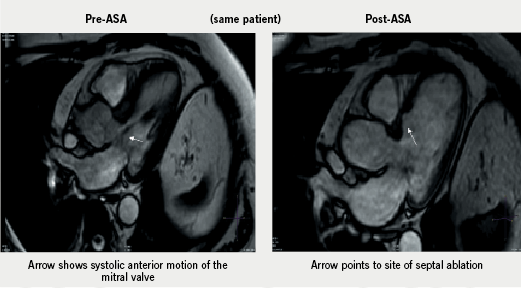
It is likely that cardiac magnetic resonance (CMR) will provide a complementary methodology for measuring the septal reduction post-procedure (figure 1). One of the attractions of ASA is that repeat procedures are well tolerated and easy to perform if the symptomatic response to a first procedure is inadequate, and we have found repeat procedures to be of value in terms of reducing symptoms and reducing gradient.
Early mortality post-ASA has fallen over the past decade. A systematic review of 42 published studies3 showed that early mortality (within 30 days) was 1.5% (0.0–5.0%) and late mortality (>30 days) was 0.5% (0.0–9.3%). ASA has undergone several changes since its introduction in 1995,6 with the use of myocardial contrast echocardiography, slow versus bolus ethanol injections and use of a reduced amount of ethanol, which reduces the procedure-related complications but may account for repeat procedure rates. In our series of 56 patients, in-hospital mortality was 0% and with a longer-term (mean follow-up 1.72 ± 2.41 years) survival of 95% (three deaths). Of the three patients who died, only one was related to HCM.
However, in a recent publication, Ten Cate et al.7 raised concerns regarding higher cardiac mortality and arrhythmias post-ASA (91 patients) versus surgical myectomy (40 patients) over a 5.4 ± 2.5 year follow-up. But the authors acknowledge the fact that in their study, which included patients from 1999–2007, the use of large amounts of ethanol during the early years may have led to larger myocardial infarctions, accounting for the excess post-procedure complications, which included arrhythmias. In addition, this was a high-risk group with 11% of patients undergoing ASA having co-existent comorbidities that made them unsuitable for surgery.
Perhaps the most interesting aspect of Hamid et al.’s work is that they have found no evidence of either significant arrhythmia or any evidence of left ventricular dilatation or remodelling as a consequence of the iatrogenic myocardial infarction induced by ASA. Conventionally, these potential risks following ASA have been argued as indicating the superiority of the surgical technique as ‘the gold standard’2 for reducing the left ventricular outflow tract (LVOT) gradient in patients with obstructive HCM. However, as experience with ASA develops in centres with expertise in percutaneous intervention for structural heart disease, as published reports such as those of Hamid et al. continue to show both efficacy and safety, and as the power of patient preference for ASA over surgery increases (89% in a recent series7), the surgical ‘gold standard’ may come under increasing scrutiny.
Conflict of interest
None declared.
Editors’ note
See also the article by Hamid et al. on pages 233–7 of this issue.
References
- Teare D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J 1958;20:1–8. (doi: 10.1136/hrt.20.1.1)
- Maron BJ, McKenna WJ, Danielson GK et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 2003;42:1687–713. (doi: 10.1016/S0735-1097(03)00941-0)
- Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol 2006;19:319–27. (doi: 10.1111/j.1540-8183.2006.00153.x)
- Maron MS, Olivotto I, Betocchi S et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med 2003;348:295–303. (doi: 10.1056/NEJMoa021332)
- Elliott PM, Gimeno JR, Tome MT et al. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J 2006;27:1933–41. (doi: 10.1093/eurheartj/ehl041)
- Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet 1995;346:211–14. (doi: 10.1016/S0140-6736(95)91267-3)
- Ten Cate FJ, Soliman OI, Michels M et al. Long-term outcome of alcohol septal ablation in patients with obstructive hypertrophic cardiomyopathy: a word of caution. Circ Heart Fail 2010;3:362–9. (doi: 10.1161/CIRCHEARTFAILURE.109.862359)
