Troponin levels are used in the diagnosis of acute coronary syndromes (ACS), however, levels may be elevated in many other conditions. A significant proportion of patients with stable heart failure (HF) have detectable levels of troponin using standard assays, however, the incidence of detectable levels of high-sensitivity troponin T (hsTnT) in HF patients is not extensively studied.
As part of a trial assessing vascular function in stable HF patients, 32 subjects had hsTnT levels measured at baseline using a multi-channel analyser (Roche E Module). At baseline, 27 (84.4%) patients had detectable levels of hsTnT (median 13.8 ng/L, range 9.2–21.4): 12 (75%) patients in the non-ischaemic group and 15 (94%) in the ischaemic group. A total of 14 (43.8%) patients had levels above the 99th percentile of the normal range.
The majority of patients with stable HF will have detectable levels of troponin T using new high-sensitivity assays. A significant proportion of these will be above the cut-off point used for diagnosis of ACS. If these patients present to hospital, modest elevations in hsTnT do not necessarily indicate recent ACS, and serial measurements should be undertaken if clinically indicated.
Introduction
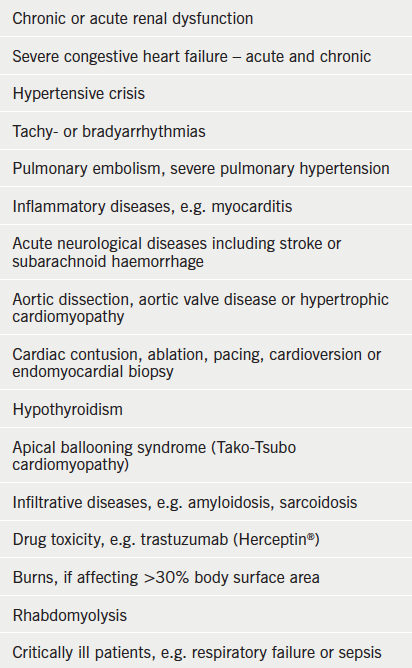
Troponins are proteins found within cardiac and skeletal muscle fibres with the physiological role of muscle fibre contraction by allowing cross bridging between actin and myosin filaments. In certain situations these intracellular molecules can be detected in the plasma, indicating loss of integrity of the cell membrane. In most cases this is due to cardiac ischaemia or infarction, and troponins have a well-established role in the diagnosis and risk stratification of acute coronary syndromes (ACS).1 However, many other cardiac and non-cardiac conditions may result in detectable levels of cardiac troponins in plasma (box 1).
Mechanistically, these low level elevations may signify leakage of the cytosolic pool of troponin during reversible injury or cell stretch. Ischaemia may be caused by a mismatch between oxygen supply and demand at a cellular level. Wall stress may be increased by chamber dilation and elevated filling pressures resulting in increased oxygen demands, while oxygen supply is reduced by factors such as anaemia, hypotension and reduced gas transfer. Progressive apoptotic and necrotic cell death have also been implicated in heart failure (HF) due to influences such as inflammatory cytokines and oxidative stress, which have a direct cytotoxic effect, although some evidence suggests that wall stress can lead directly to myocardial cell death independently of ischaemia.2 Another theory suggests that intracellular proteolysis may allow release of troponin fragments, which may have affinity for immunoassays.3
Several studies have reported elevated levels of cardiac troponins in patients with HF in the absence of ACS,4 and, as in ACS, degree of troponin elevation correlates with worse prognosis.5,6 A recent review of troponin elevations in HF found an overall hazard ratio of 2.85 (95% confidence interval [CI] 2.02, 4.03) for mortality in patients with elevated troponin.7 Table 1 summarises significant studies of troponin levels in HF patients.
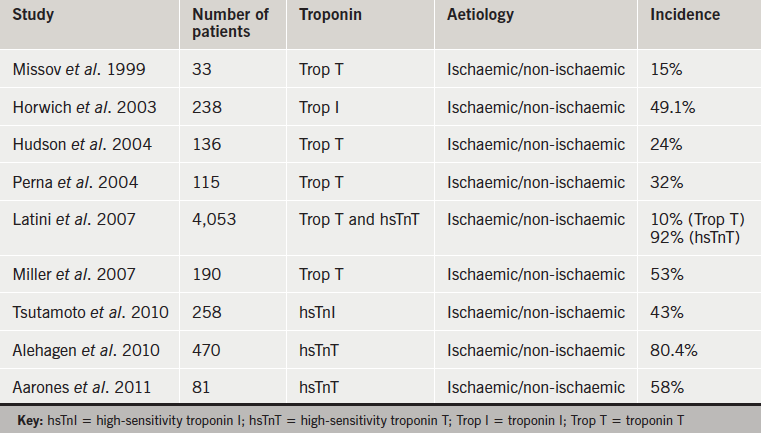
More recently, the need for a biomarker with adequate precision and a low coefficient of variation at the upper limit of normal in the reference population, has prompted development of a new generation of troponin assay. A fourth-generation high-sensitivity troponin T (hsTnT) assay developed by Roche can now detect troponin T levels down to 3 ng/L with a 99th percentile value of 14 ng/L in the normal population.8 Tentzeris et al. found hsTnT to be elevated in 58% of patients with chronic stable heart failure,9 and in a Japanese study of 85 patients with dilated cardiomyopathy, hsTnT was elevated in 54% of patients (compared with only 5% with elevation of conventional troponin T), and again, was an independent predictor of worse prognosis.10 Elevated troponin levels in HF patients tend to be more common when measured using high-sensitivity assays compared with earlier generation assays, although there is wide variation between studies (11–54% for low-sensitivity assays and 43–92% for high-sensitivity assays).6 Patients with HF tend to have multiple hospital admissions and may have troponin levels measured on admission. The finding of a raised troponin may suggest possible ACS and lead to unnecessary and potentially harmful treatment. The frequency and degree of troponin elevation in stable HF patients without ACS, particularly using new high-sensitivity assays is, therefore, of interest.
Materials and methods
As part of a study assessing the effect of statin therapy on biomarkers of HF, we studied 32 outpatients with stable HF. Patients were recruited from heart failure clinics within the Belfast Health and Social Care Trust.
Inclusion criteria were:
- diagnosis of HF with an ejection fraction <45% determined by non-invasive imaging (2D echocardiography, myocardial perfusion scintigraphy or cardiac magnetic resonance imaging [MRI]) within previous 18 months
- receiving stable medical therapy for treatment of HF, including loop diuretics, angiotensin-converting enzyme (ACE) inhibitors or angiotensin-receptor blockers (ARBs), beta blockers and spironolactone, as tolerated
- able to give informed consent and to attend for follow-up appointments
- over 18 years of age.
Exclusion criteria were:
- history of uncontrolled hypertension (blood pressure [BP] >140/90 mmHg)
- receiving the thienopyridine derivative clopidogrel, due to inhibitory effect on platelet assays
- abnormal liver function (defined as aspartate aminotransferase [AST] or alanine aminotransferase [ALT] >3 times upper limit of normal)
- previously documented adverse reaction to statin therapy or hypersensitivity to simvastatin or any of the excipients
- currently taking potent CYP3A4 inhibitors (e.g. itraconazole, ketoconazole, HIV protease inhibitors, erythromycin, clarithromycin) or ciclosporin, gemfibrozil or >1 g/day niacin, due to statin interaction
- females were excluded if they were pregnant or lactating.
HsTnT was analysed on the Roche Cobas E602 multi-channel analyser (Immunoassay E Module) at the Belfast HSC Trust laboratories.
Results
In total, 32 patients were recruited. The majority were male (78%). Mean age was 63.5 years (standard deviation [SD] 10.9). Mean body mass index (BMI) was 30.4 (SD 6.0) and seven patients had a BMI >35 kg/m2. Six patients (20%) were diabetic and 12 (37.5%) had a history of atrial fibrillation. Four (12.5%) patients had significant valvular heart disease (classified as at least moderate valve regurgitation or stenosis). The mean serum creatinine was 103 mmol/L (SD 28.6). There were 14 patients (43.8%) taking antiplatelet therapy (all aspirin) and 14 (43.8%) were taking warfarin. Patients were on typical HF therapy to include beta blockers (90.6%), ACE inhibitors or ARBs (96.9%) and spironolactone (56.3%). Overall, 16 patients (50%) had non-ischaemic cardiomyopathy. All patients were in New York Heart Association (NHYA) class II (46.9%) or III (53.1%) and mean ejection fraction was 31% (SD 9.6). Eight patients (25%) had an implantable cardiac device.
HsTnT levels were available for 31 patients. The frequency histogram for baseline troponin levels in the patient group is shown in figure 1.
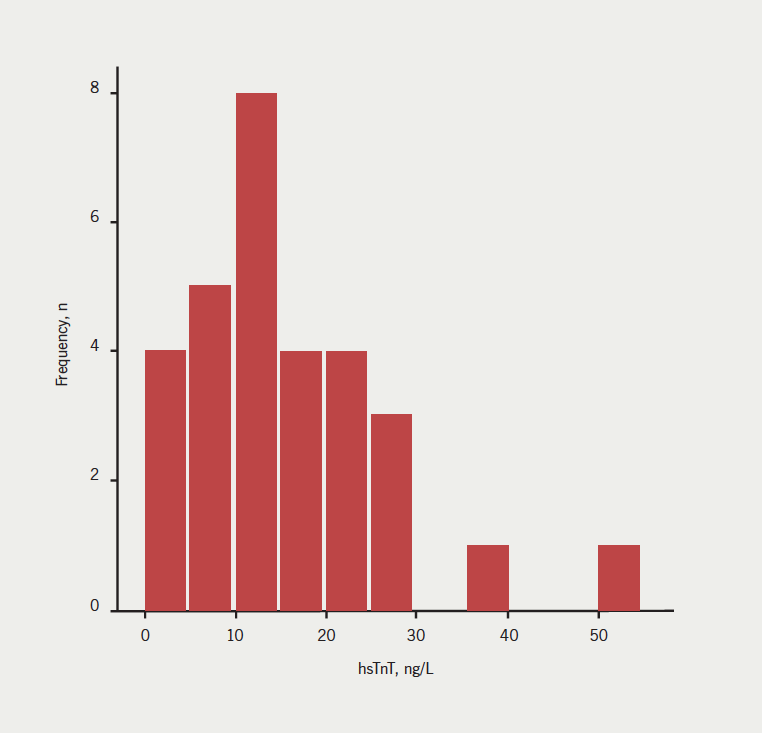
Median hsTnT was 13.9 ng/L (interquartile range [IQR] 9.2–21.6): 27 (84.4%) patients had detectable levels of hsTnT in plasma and 14 (43.8%) patients had levels above the 99th percentile of the normal range (commonly used as the cut-off for diagnosis of ACS).
As cardiac troponins are generally used in the diagnosis of ischaemic cardiac syndromes, comparison was made between hsTnT levels in patients with ischaemic and non-ischaemic cardiomyopathies at baseline. There were 12 (75%) patients in the non-ischaemic group and 15 (94%) in the ischaemic group with detectable levels of hsTnT at baseline, while seven (44%) patients in the non-ischaemic group and six (38%) in the ischaemic group had levels greater than 14 ng/L. These differences were not statistically significant (p=0.33 for any detectable level and p=1.0 for level >14 ng/L). Serum levels were similar in ischaemic (median 13.93 ng/L, IQR 11.8–20.2) and non-ischaemic (median 13.71 ng/L, IQR 4.6–26.3) groups (p=0.80).
In a subgroup of 16 patients, hsTnT levels were repeated after a period of four to six weeks. The median change in hsTnT was –1.2 ng/L (IQR –7.0 to +1.4) and this was not statistically significant (p=0.177).
Discussion
Cardiac troponin levels are known to be elevated in the HF population. The proportion of HF patients with baseline elevations varies in studies depending on population subtypes, troponin assay used, and cut-off point defined for raised levels. In this study, 84.4% of patients had detectable levels in plasma and 43.8% had levels above the 99th percentile for the normal range, which is a higher proportion than that described in many previous studies of patients with both acute and chronic HF,3 even when using a high-sensitivity assay. One previous study, using a high-sensitivity troponin assay, described a similar proportion of patients with detectable circulating troponin levels (92%), although this was using a pre-commercial assay.11 Interestingly, there was no significant difference between baseline levels of hsTnT in patients with ischaemic (13.9 ng/L, 95% CI 11.8–20.2) and non-ischaemic (13.7 ng/L, 95% CI 4.6–26.3) aetiologies (p=0.8). The mechanisms hypothesised to account for troponin elevations in HF, other than ischaemia, include neurohormonal activation, cytotoxic effect of inflammatory cytokines, cell stretch and the effect of oxidative stress, which may lead to chronic low-level troponin elevations. It could be hypothesised, therefore, that in clinically stable disease, the final mechanisms leading to chronic troponin elevation are similar in ischaemic and non-ischaemic aetiologies. Certainly, a history of major epicardial vessel ischaemia did not result in greater troponin elevations in this group.
In this study, hsTnT levels appeared to be stable over a relatively short period of time. This is important to consider when the diagnosis of infarction is based, not only on elevated levels, but also on demonstrating a delta change in troponin levels. Elevations due to HF should remain relatively stable over short periods of time, with a median change of less than 10% demonstrated in this study.
Limitations
The findings of this study are limited by the relatively small sample size. Also, the patients studied had significant comorbidity, both cardiac and non-cardiac, which may have contributed to the finding of elevated troponin. However, HF patients typically have complex medical problems, and the patients in this study had similar levels of arrhythmia and valvular disease to the general HF population.
Summary
Almost half of patients studied had baseline hsTnT levels above the standard cut-off point used for the diagnosis of ACS. As patients with HF often have co-existing coronary disease and may present with chest pain of possible ischaemic origin, it is important to recognise that modest elevations of hsTnT may not necessarily indicate a recent ACS. Although the guidelines state that a characteristic rise and fall in biomarker levels is necessary for the diagnosis of a myocardial infarction,12 in practice, serial measures are not always performed. In HF patients, if the clincial presentation is unclear, it may be prudent to await repeat measurements showing a significant delta change in troponin levels before prescribing potentially harmful ACS therapies.
Source of support
This research was supported by a Research and Development grant from the Public Health Agency Northern Ireland.
Conflict of interest
None declared.
Key messages
Troponin levels measured using new generation high-sensitivity assays are detectable in the majority of patients with stable heart failure of both ischaemic and non-ischaemic aetiologies
A significant proportion will have levels above the cut-off point used for diagnosis of acute coronary syndrome
Levels appear to be stable, at least over relatively short periods of time
References
1. Hamm CW, Bassand J-P, Agewall S et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999–3054. http://dx.doi.org/10.1093/eurheartj/ehr236
2. Januzzi J, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart failure section. Eur Heart J 2012;33:2265–71. http://dx.doi.org/10.1093/eurheartj/ehs191
3. Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O’Connor CM, Felker GM. Troponin elevation in heart failure: prevalence, mechanisms, and clinical implications. J Am Coll Cardiol 2010;56:1071–8. http://dx.doi.org/10.1016/j.jacc.2010.06.016
4. Wang TJ. Significance of circulating troponins in heart failure. Circulation 2007;116:1217–20. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.721845
5. Sato Y, Yamada T, Taniguchi R et al. Persistently increased serum concentrations of cardiac troponin T in patients with idiopathic dilated cardiomyopathy are predictive of adverse outcomes. Circulation 2001;103:369–74. http://dx.doi.org/10.1161/01.CIR.103.3.369
6. Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 2003;108:833–8. http://dx.doi.org/10.1161/01.CIR.0000084543.79097.34
7. Nagarajan V, Hernandez A, Tang WHW. Prognostic value of cardiac troponin in chronic stable heart failure: a systematic review. Heart 2012;98:1778–86. http://dx.doi.org/10.1136/heartjnl-2012-301779
8. Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 2010;56:254–61. http://dx.doi.org/10.1373/clinchem.2009.132654
9. Tentzeris I, Jarai R, Farhan S et al. Complementary role of copeptin and high-sensitivity troponin in predicting outcome in patients with stable chronic heart failure. Eur J Heart Fail 2011;13:726–33. http://dx.doi.org/10.1093/eurjhf/hfr049
10. Kawahara CH, Tsutamoto T, Nishiyama K et al. Prognostic role of high-sensitivity cardiac troponin T in patients with nonischemic dilated cardiomyopathy. Circ J 2011;75:656–61. http://dx.doi.org/10.1253/circj.CJ-10-0837
11. Latini R, Masson S, Anand IS et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007;116:1242–9. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.655076
12. Thygesen K, Alpert JS, Jaffe AS et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–67. http://dx.doi.org/10.1093/eurheartj/ehs184
