Major new trials reported at the American Heart Association 2007 Scientific Sessions, held in Orlando, Florida, US, on November 3rd–7th, showed mixed results for the new antiplatelet agent, prasugrel, and gave renewed hope for the high-density lipoprotein raising field. But there was disappointment regarding the use of statins in heart failure and beta blockers in general surgery.
Prasugrel lowers events but increases bleeding compared with clopidogrel in PCI patients
The new antiplatelet agent, prasugrel, reduced ischaemic events compared with clopidogrel but at the cost of an increase in major bleeding in the TRITON-TIMI 38 trial in acute coronary syndrome (ACS) patients scheduled for percutaneous coronary intervention (PCI). Overall mortality did not differ significantly between the two groups.
In the study, which has also been published in the New England Journal of Medicine, prasugrel prevented 23 myocardial infarctions (MIs) for every 1,000 patients treated but caused an excess of six non-CABG (coronary artery bypass grafting)-related TIMI major bleeds. The authors conclude that: “When considering the choice of antiplatelet regimens for the treatment of patients with ACS who are undergoing PCI, clinicians need to weigh the benefits and risks of intensive inhibition of platelet aggregation”.
In an accompanying editorial in the New England Journal of Medicine, Dr Deepak Bhatt (Cleveland Clinic, Cleveland, US) notes that: “Prasugrel would probably benefit patients with ACS who are undergoing PCI and who are at high risk of ischaemic events and low risk for bleeding, though those with a lower risk for ischaemic events and a high risk of bleeding may be better served with clopidogrel”.
The TRITON-TIMI 38 trial randomised 13,608 moderate-to high-risk ACS patients scheduled for PCI to receive prasugrel (60 mg loading dose and then 10 mg daily maintenance dose) or clopidogrel (300 mg/75mg) for six to 15 months. Presenting the results at the meeting, Dr Elliott Antman (Brigham & Women’s Hospital, Boston, US) focused on the net clinical benefit end point of all-cause mortality, MI, stroke and major bleeding, which showed a significant benefit for prasugrel (table 1).
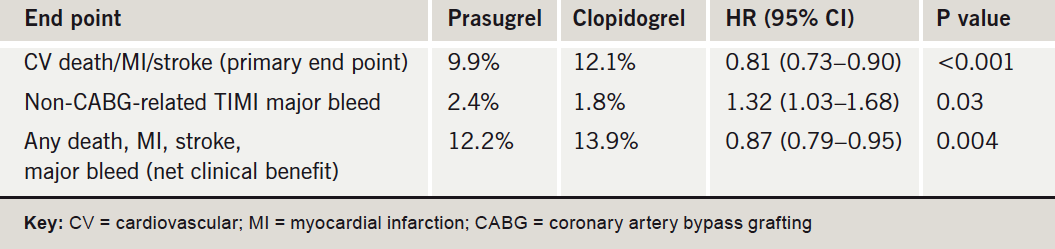
Subgroup analyses showed that in patients with a history of stroke/transient ischaemic attack, the bleeding risk outweighed the benefits of prasugrel versus clopidogrel. And for two other groups – those older than 75 years or under 60 kg in weight, the net clinical benefit appeared neutral. Dr Antman suggested that in these “neutral” groups, a reduction in the maintenance dose of prasugrel may produce better results. But he added: “In the remaining 80% of the population enrolled in this study, there was a strong net clinical benefit of prasugrel”.
Commenting upon the results of TRITON-TIMI-38, Dr Clive Weston (Singleton Hospital, Swansea) said: “It will come as no surprise to practising clinicians that a treatment that is shown to be more effective, in this case with respect to preventing the combination of cardiac death, heart attack and stroke following presentation with acute coronary syndrome, should also produce more unwanted events, in this case bleeding. All our interventions are predicated on the belief that the net effect is beneficial – that on balance we do more good than harm. This appears to be what the trial is telling us about prasugrel.
“My initial impression after reading the study results are that the earlier suggestion that prasugrel (at the dose chosen in this trial) has a more predictable antiplatelet effect than conventional doses of clopidogrel has been confirmed, because this would account for both the beneficial and adverse effects. The remarkable thing is that the size both of the benefit and the added risk, in relative and absolute terms, is strikingly similar to that reported for clopidogrel in the CURE study. It was upon such differences that the widespread use of clopidogrel in ACS was endorsed by the National Institute of Health and Clinical Excellence.”
POISE – should beta blockers be given in non-cardiac surgery?
The use of extended-release metoprolol in patients undergoing non-cardiac surgery was associated with a reduced risk of myocardial infarction (MI), but an increased risk of stroke and death in the POISE (PeriOperative Ischaemic Evaluation) study.
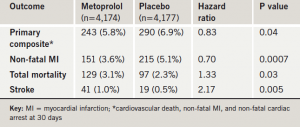
Results of the trial (table 1), the first large randomised study to investigate whether beta blockers should be used routinely in surgery patients to prevent cardiac events, suggested that for every 1,000 patients treated, the beta blocker would prevent 15 MIs, at the cost of eight deaths and five disabling strokes. Dr Philip Devereaux (McMaster University, Hamilton, Canada), who presented the study, said that physicians should weigh the potential risks and benefits before deciding whether to use beta blockers peri-operatively.
Discussant of the trial, Dr Judith Hochman (New York University School of Medicine, New York, US) said that based on these results, she would not recommend routine use of beta blockers in this setting. But she stressed that these results do not apply to patients already taking beta blockers undergoing surgery (who were excluded from POISE) or to patients in whom a physician had planned to prescribe a beta blocker within 30 days of surgery.
Dr Devereaux reported that one million non-cardiac surgery patients suffer a major peri-operative cardiovascular event each year, and that guidelines currently suggest that beta blockers should be started before elective surgery –– particularly in high-risk patients – with the dose titrated to achieve a resting heart rate of 50–60 bpm.
POISE enrolled 8,351 patients undergoing non-cardiac surgery with, or at risk of, atherosclerotic disease who were randomised to metoprolol CR or placebo, started two to four hours pre-operatively and continued for 30 days. Metoprolol was given at a dose of 100 mg in the pre-operative period, and 200 mg in the post-operative period. Doses were not titrated and the drug was only stopped if blood pressure dipped below 100 mmHg systolic.
Dr Hochman said a number of issues about the trial still needed to be addressed. These included the dose of metoprolol used, which might have been too high, and the lack of titration. She suggested that a systolic blood pressure of 100 mmHg may be too low for some patients, such as those prone to hypotension and the elderly.
AF-CHF: rate control just as good as rhythm control for AF in heart failure
The simpler strategy of rate control was just as effective as rhythm control for treating atrial fibrillation (AF) in patients with congestive heart failure (CHF) in the AF-CHF trial. Lead investigator, Dr Denis Roy (Montreal Heart Institute, Montreal, Canada), concluded: “A routine strategy of rhythm control cannot be advocated in heart failure patients with AF. Rate control is a simpler strategy, with less cardioversion and less hospitalisations.”
Discussant of the trial, Dr Rodney Falk (Boston University School of Medicine, Boston, US) said: “The AF-CHF trial results have provided us with another extremely important step forward in the understanding and treatment of this common arrhythmia. We can now manage the common clinical situation of co-existing AF and systolic heart failure by rate control alone, without concern that we may worsen the clinical condition”.
Dr Roy explained that no previous trial looking at rhythm versus rate control in AF had included patients with heart failure. The AF-CHF study randomised 1,376 patients to rhythm or rate control. The rhythm control patients received anti-arrhythmic therapy with amiodarone (and sotalol and dofetilide in selected cases) and electrical cardioversion if still not in sinus rhythm after six weeks. Those in the rate control arm received titrated doses of beta blockers and digitalis.
There was no difference in the primary end point of cardiovascular mortality between the two groups. Total mortality, worsening CHF and stroke were also similar between the two groups. Hospitalisation was higher in the rhythm group, (accounted for mainly by AF and bradyarrhythmias) and, as expected, cardioversions were also much higher in the rhythm control group (39% versus 8%).
Simvastatin increases sleep problems?
Simvastatin, but not pravastatin, appears to interfere with sleep quality, according to the UCSD Statin Study. In the trial, patients taking simvastatin exhibited significantly worse subjective sleep, relative to either placebo or pravastatin, despite comparable sleep ratings at baseline. Presenting the study, Dr Beatrice Golomb (University of California, San Diego, USA) said the findings were important as many patients are currently being switched to simvastatin.
The current study evaluated the non-cardiac effects of statins in 1,016 patients in whom statin therapy was considered optional and who were randomised to receive 40 mg of pravastatin, 20 mg of simvastatin or placebo and followed for six months. Sleep was a prespecified secondary end point. At baseline, the three randomised groups were equivalent in terms of their sleep quality and sleep problems, but on follow-up the simvastatin group reported significant worsening relative both to the placebo group and the pravastatin group in both the sleep-quality outcome and the sleep-problem outcome. Pravastatin did not differ significantly from placebo on either of these measures.
Torcetrapib had toxicity unrelated to HDL-raising mechanism
New data from trials with the now discontinued high-density lipoprotein (HDL)-increasing drug, torcetrapib, have shown that it stimulates aldosterone, which may possibly account for its adverse outcomes. In addition, patients with the largest HDL rises showed benefits with the drug, suggesting that the HDL produced was functional. These observations raise hopes that other drugs which raise HDL by the same mechanism as torcetrapib may still be effective.
Final results of the ILLUMINATE (Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events) trial of torcetrapib were presented by Professor Philip Barter (Heart Research Institute, Sydney, Australia). The study was stopped last December because of a higher rate of both deaths and cardiovascular events in the torcetrapib group.
Professor Barter commented that the observation that torcetrapib increases aldosterone levels could explain some of the adverse clinical results with the drug. “Several other CETP inhibitors in early development do not appear to have this effect on aldosterone, which gives us hope that this class of drugs may still be successful,” he said. “This class of drug has the potential to save so many lives. We should not give up on them,” he added.
The ILLUMINATE results showed that torcetrapib was associated with a decrease in serum potassium, and increases in serum sodium, bicarbonate and aldosterone, and post-hoc analyses showed an increased risk of death in patients treated with torcetrapib whose reduction in potassium or increase in bicarbonate was greater than the median change, Professor Barter reported.
The data suggesting that the HDL produced by torcetrapib does have protective properties come from a post-hoc exploratory analysis of the ILLUMINATE trial. This showed that rates of coronary heart disease death/myocardial infarction were lowest in patients with the largest increases in HDL levels in patients receiving torcetrapib.
Similar data were also presented from the ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation) study, showing that increasing levels of HDL from torcetrapib treatment were associated with a beneficial impact on atherosclerotic plaque progression. In that study, patients with reduced potassium levels did not gain the same benefits, suggesting that stimulation of aldosterone may be mitigating the benefit of increasing HDL.
In the ILLUMINATE paper, published in the New England Journal of Medicine, Professor Barter and his colleagues conclude: “Our study neither validates nor invalidates the hypothesis that raising levels of HDL cholesterol by the inhibition of CETP may be cardioprotective. Thus, the possibility that the inhibition of CETP may be beneficial will remain hypothetical until it is put to the test in a trial with a CETP inhibitor that does not share the off-target pharmacologic effects of torcetrapib”.
New data reassuring on safety of drug-eluting stents
New data from a large US observational study suggest that drug-eluting stents are just as safe as bare-metal stents. The study included 11,516 patients who were treated with drug-eluting stents and 6,210 patients who were treated with bare-metal stents in non-US government hospitals in Massachusetts, USA, between 2003 and 2005.
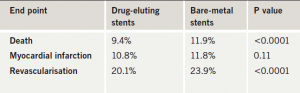
Presenting the data, Dr Laura Mauri (Brigham and Women’s Hospital, Boston, US) said that after two years of follow-up, rates of myocardial infarction were not statistically different between the two groups; however, rates of death, as well as rates of revascularisations were lower in patients who had received a drug-eluting stent (table 1).
CORONA: no firm benefit of statins in heart failure
The first major trial of a statin (rosuvastatin) in heart failure has failed to show a significant reduction in the primary end point – a composite of cardiovascular death/non-fatal myocardial infarction/stroke. But CORONA (Controlled Rosuvastatin Multi-national study in Heart Failure) did suggest a reduced rate of cardiovascular hospitalisations with the drug.
The study randomised 5,011 older patients (average age 73 years) with moderate-to-severe heart failure of ischaemic origin to rosuvastatin or placebo. Over a median follow-up of 33 months, there were no significant differences in the primary end point or in all-cause mortality or the rate of coronary events. The only observed clinical difference was in cardiovascular hospitalisations, which were reduced from 25% in the placebo group to 22.9%
in the rosuvastatin group.
Treatment with the statin was not associated with an increase in any adverse events, Dr Åke Hjalmarson (Salhgrenska University Hospital, Göteborg, Sweden) reported. In an editorial accompanying publication of the study in the New England Journal of Medicine, Dr Frederick Masoudi (University of Colorado, Denver, US) suggests that statins may not improve cardiovascular outcomes noticeably in an older population who are at higher risk for competing events.
Genetic guidance helps find correct warfarin dose
Using genetic tests to guide warfarin dosing reduced the number of dose changes needed to get to the correct INR (international normalized ratio) in the Couma-Gen study. While the primary end point of percentage out- of-range INRs was not reduced in the whole population, genetic testing did appear to improve this end point in certain patient groups.
Presenting the study, Dr Jeffrey Anderson (University of Utah, Salt Lake City, USA) explained that several gene variants have been discovered which affect inter-individual dose variability of warfarin. The Couma-Gen study randomised 206 patients being initiated on warfarin to pharmacogenetic guided or standard dosing. The genetic tests were performed on buccal swab samples, with results available one hour later. The results were then entered, along with data on weight, age and sex, into a computer program to obtain an individualised dose for each patient. Results showed that age, sex and weight explained 12% of the variability of warfarin dosing, while genetics explained 35% of the variability.
During the three-month follow-up, out-of-range INRs did not differ significantly between the two arms in the whole population, but subgroup analysis suggested that those with the wild-type genotype (no genetic variants) and carriers of multiple variant alleles showed promising reductions in out-of-range INRs with pharmacogenetic guidance.
Dr Anderson explained that the average patient has one genetic variant and that these patients are probably well suited to the standard warfarin dose. But he added that the standard dose of warfarin appears to be too much for wild-type patients and too little for patients with more than one genetic variant.
He stressed that larger trials of genetic-based warfarin dosing are needed, and are now being planned.
No role for T-wave alternans testing in selecting patients
for ICDs
T-wave alternans testing, which measures alterations in the T-wave of the electrocardiogram (ECG), did not predict life-threatening ventricular tachyarrhythmic events in post-myocardial infarction patients with left ventricular ejection fractions of less than 30% in the MASTER I trial.
Presenting the trial, Dr Theodore Chow (Lindner Center at the Christ Hospital, Cincinnati, USA) explained that it was hoped that T-wave alternans testing would help select those patients who currently fit MADIT-2 criteria and would gain most from having an implantable cardioverter defibrillator (ICD) implanted. But although patients with an abnormal T-wave alternans test were shown to have a higher risk of all-cause death in this study, they did not have a higher rate of life-threatening ventricular tachycardia as judged by ICD shocks, or an increased risk of sudden cardiac death.
The MASTER I trial included 575 patients who met the MADIT-2 indication for ICD implantation who underwent T-wave alternans testing and then had an ICD implanted. After two years follow-up, the primary end point of life-threatening ventricular tachyarrhythmic events (as assessed by ICD shocks) was not significantly different between patients with negative and non-negative T-wave alternans tests. Mortality results showed that all-cause deaths were increased in the patients with non-negative tests, but this appeared to be accounted for mainly by an increase in non-cardiac deaths.
Discussant of the study, Dr Alan Kadish (Northwestern University Medical School, Chicago, US) noted that previous studies had suggested arrhythmic events could be predicted with T-wave alternans testing. The reasons why this study had not shown such results are unknown.
Fenofibrate reduces need for laser treatment
for diabetic retinopathy
Treatment with fenofibrate in individuals with type 2 diabetes reduces the need for laser treatment for diabetic retinopathy, according to new results from the FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) trial. Presenting the results, Professor Tony Keech (University of Sydney, Australia) noted that diabetic retinopathy has become the leading cause of blindness in working-age adults, and that pathological changes associated with the condition are strongly related to hyperglycaemia in type 2 diabetes.
The FIELD trial, first reported in 2005, failed to show a significant reduction of fenofibrate on the primary end point (death from coronary heart disease or non-fatal myocardial infarction) in patients with diabetes. In the retinopathy substudy, retinal photography was conducted in 1,012 patients. This showed that after an average follow-up of five years, fenofibrate reduced the frequency of first laser treatment for macular oedema and for proliferative diabetic retinopathy. However, the actual number of events was small (23 surgeries in the placebo group versus five in the fenofibrate group).
Professor Keech reported that fenofibrate did not produce clinically important differences in high-density lipoprotein cholesterol levels, suggesting that its mechanism of benefit in diabetic retinopathy must be conferred mainly by other means. He concluded: “The retinal benefits argue for consideration of using fenofibrate in the management of diabetic eye disease, and should be considered in the context of other effects reported with fenofibrate in the FIELD study”. But an editorial accompanying publication of the retinopathy substudy in the Lancet, says: “ The mechanisms by which fenofibrate exerts its reported benefits are far from being elucidated…further clinical and experimental studies are needed before fenofibrate can be launched as a new tool in the management of diabetic retinopathy.”
Computed tomography angiography shows diagnostic accuracy but discussant urges caution
64-slice computed tomography angiography (CTA) has comparable diagnostic accuracy to quantitative coronary angiography in patients without highly calcified vessels and suspected coronary artery disease, according to the results of the CORE-64 (Coronary Evaluation Using Multi-detector Spiral Computed Tomography Angiography using 64 Detectors) trial.
The trial included 291 patients over the age of 40 years, who were referred for a diagnostic catheterisation for suspected coronary artery disease (CAD). All patients underwent a 64-slice CTA and standard coronary angiography and were then followed for 30 days. Results showed a high degree of agreement between the ability of quantitative CTA and standard quantitative coronary angiography to identify important stenoses. Presenter, Dr Julie Miller (Johns Hopkins University, Baltimore, US) concluded: “In patients with suspected CAD and a calcium score of less than 600, 64-detector CTA can be used to assess the presence of significant CAD and the potential need for coronary revascularisation. Our interpretation of this analysis is that multidetector CT will become an integral part of the diagnostic algorithm in patients with coronary artery disease”.
But in his discussion of the study, Dr Michael Lauer (US National Heart, Lung, and Blood Institute) criticised the way new imaging technologies were being brought to market without any real outcomes data. “We know nothing about the diagnostic accuracy of CT angiography in the real word, we have minimal data on its prognostic value, but most important, we have no evidence that the use of CT angiography saves lives or prevents heart attacks. CTA is a technology with enormous promise. It may save lives, but before we get carried away, we have to prove it,” he argued. Dr Lauer added that the technology could also carry a risk that was currently unknown. “By letting this technology loose upon the public we may be causing thousands of preventable cancers,” he said.
HIJ-CREATE: no benefit of ARB instead of ACE inhibitor for hypertension
Replacing an angiotensin-converting enzyme (ACE) inhibitor with an angiotensin receptor blocker (ARB) in the multiple drug treatment of hypertension in patients with heart disease made no difference to cardiovascular clinical outcomes, in the HIJ-CREATE (The Heart Institute of Japan – Candesartan Randomized trial for Evaluation in Coronary Artery Disease) study.
The study, presented by Dr Hiroshi Kasanuki (Tokyo Women’s Medical University, Japan) involved 2,049 patients with hypertension and heart disease who were given standard hypertension therapy and were randomised to receive either an ACE inhibitor or the ARB, candesartan.
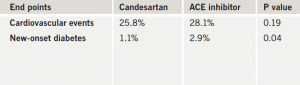
The primary end point – major adverse cardiac events – was not significantly different between the ARB- and ACE-inhibitor-based strategies over the four-year follow-up (table 1). But among those patients who had reduced renal function, the primary end point was significantly reduced in the candesartan group. The ARB was also associated with a significantly lower risk of new-onset diabetes in the study.
Discussing the trial, Dr Beatriz Rodriguez (University of Hawaii, USA) noted that the results were consistent with those of the VALIANT study in myocardial infarction patients, which also concluded that ARBs are similarly effective to ACE inhibitors in reducing cardiovascular events.
Correction
We wish to correct a statement made in a recent supplement to the journal: ‘The debate for and against: should cholesterol targets be 4 and 2 mmol/L?’ (Br J Cardiol 2007; 14(suppl 2):S1). The text stated: “The SIGN guidelines have also endorsed the generalised ‘5 and 3’ targets while saying that those with established CHD should be considered for intensive statin therapy”.
In fact, for primary prevention the guideline text reads: “All adults over the age of 40 years who are assessed as having a ten year risk of having a first cardiovascular event ≥20% should be considered for treatment with simvastatin 40 mg/day following an informed discussion of risks and benefits between the individual and responsible clinician”. There is no mention of targets.
For secondary prevention the full text reads: “While patients with established symptomatic cardiovascular disease should be considered for intensive statin therapy, the long term safety and cost-effectiveness of such therapy has not been established”. The words in italics were omitted from the supplement.
This was entirely unintentional and we apologise for any misinterpretation as the report represented an abbreviated overview of a lengthy and stimulating debate. The report was approved by all the debate participants prior to publication.
