Rhabdomyolysis is an uncommon but potentially serious adverse reaction associated with the use of statins. Simvastatin is metabolised by cytochrome P450 CYP3A4 and amiodarone is an inhibitor of this enzyme. Concomitant use of these drugs, especially with high doses of simvastatinm may result in myopathy. Acute renal failure as a result of rhabdomyolysis due to this aetiology is rare with only a few cases reported previously. Here, we report a case of rhabdomyolysis and acute renal failure secondary to concomitant use of simvastatin and amiodarone.
Introduction
Statins are increasingly used for primary and secondary prevention of cardiovascular disease, and in the UK simvastatin is now available as an ‘over-the-counter’ drug without prescription. Rhabdomyolysis is a rare and potentially life-threatening complication of statin therapy and with the wider use of statins there is an increased probability of drug interactions, which may increase the incidence of myopathy and rhabdomyolysis. Early recognition is essential to prevent serious consequences. We report a case of rhabdomyolysis complicated by acute renal failure associated with the concomitant use of high-dose simvastatin
and amiodarone.
Case history
A 74-year-old white male with a history of hypertension, atrial fibrillation and previous coronary artery bypass graft was admitted with diffuse muscle pain and weakness of his lower limbs. He described himself as ‘waddling like a duck’ for four days. Medication comprised of ramipril 10 mg/day, aspirin 75 mg/day, bendroflumethiazide 2.5 mg/day and simvastatin 80 mg/day. He was commenced on amiodarone 200 mg/day, a month prior to his hospital admission. Neurological examination confirmed reduced power in the right lower limb (3/5) and shoulder abduction 4/5 bilaterally. He had a waddling gait but otherwise examination was normal.
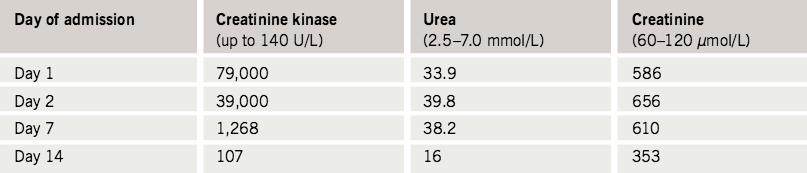
Laboratory investigations (normal range): urea 33.9 mmol/L (2.5–7.0 mmol/L), creatinine 586 µmol/L (60–120 µmol/L), thyroid-stimulating hormone 6.01 mU/L (0.1–5.0 mU/L), free thyroxine 19 pmol/L (9–28 pmol/L), alanine transaminase 433 U/L (< 41 U/L), gamma-glutamyltransferase 64 U/L (< 50 U/L) and creatinine kinase 79,000 U/L (< 160 U/L). The urine was positive for myoglobin.
Simvastatin and amiodarone were withdrawn and he received aggressive fluid resuscitation with sodium bicarbonate to alkalise the urine. His renal function improved (table 1) and he did not require dialysis. His liver function also improved. At the time of discharge from hospital, his creatinine kinase had fallen to 107 U/L and his gait improved as well.
Discussion
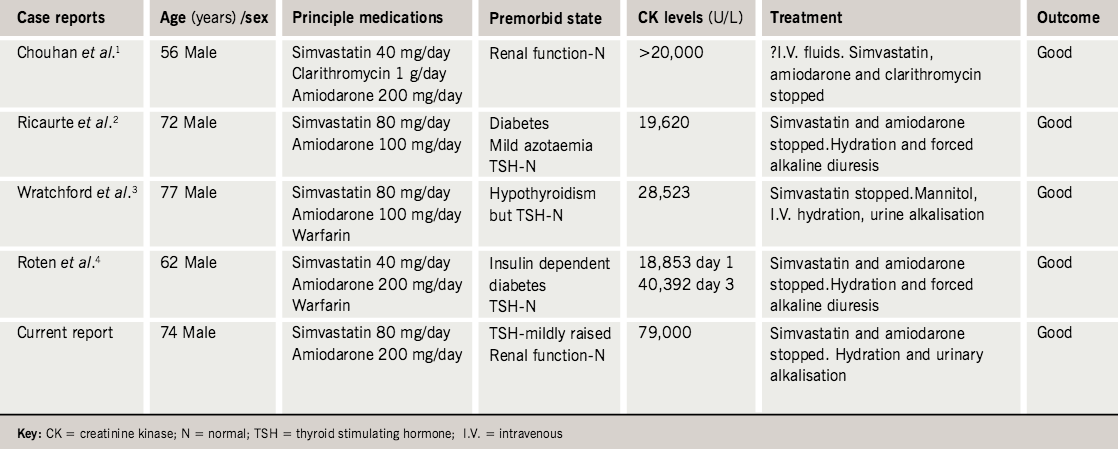
In summary, we report a case of rhabdomyolysis secondary to concomitant use of high-dose simvastatin and amiodarone. Although myopathy/rhabdomyolysis is a well-documented adverse effect of statin therapy, a literature search had revealed only a few case reports1–4 (table 2) regarding the drug interaction between statin and amiodarone, resulting in rhabdomyolysis.
Rhabdomyolysis is defined by creatinine kinase levels of more than 10 times the upper limit of normal and elevated plasma creatinine, usually associated with myoglobinuria.5 Statins reduce the risk of myocardial infarction, coronary heart disease and/or death, both in primary and secondary prevention. Myopathy occurs in 0.1–0.2% of people receiving statin therapy,6 which if left untreated will progress to rhabdomyolysis in 0.04–0.2%.7 Concurrent use of drugs that are metabolised by the enzyme cytochrome P450 3A4 increases the risk of statin-induced myopathy; one report suggests that approximately 58% of cases of statin-induced rhabdomyolysis are due to drug interactions.6 About 10–50% of patients with rhabdomyolysis develop acute renal failure.8 Advancing age, diabetes mellitus, liver disease, renal impairment, alcoholism, hypothyroidism and concurrent use of fibrates are additional risk factors associated with myopathy.6 In one study the number needed to treat (NNT) to observe one case of rhabdomyolysis was 22,727 for statin monotherapy and 484 for older patients with diabetes mellitus taking both a statin and a fibrate.9 Cerivastatin was withdrawn following a cluster of cases of rhabdomyolysis where, when combined with a fibrate, the risk was as high as one in 10.9
The classic triad of symptoms includes muscle pain, weakness and dark urine.8 The suggested mechanisms of myotoxicity with statins include depletion of metabolic intermediates, apoptotic cell death, alterations of chloride-channel conductance within the myocytes, and selenoprotein deficiency.5 The muscle pathology is characterised by loss of striations and nuclei, with regeneration in part, and no infiltration of inflammatory cells.5 The pathophysiology of myoglobinuric acute renal failure has been studied extensively in the animal model of glycerol-induced acute renal failure. The main pathophysiological mechanisms include renal vasoconstriction, intraluminal cast formation and direct haem protein cytotoxicity.10 The treatment of rhabdomyolysis includes aggressive fluid replacement and preservation of renal function. Mannitol and bicarbonate, although commonly recommended, are of unproven benefit.8
Individual statins may differ in their risk of inducing rhabdomyolysis, with some patients developing myopathy when switched from one statin to another, while others develop rhabdomyolysis when exposed to any statin.8 One recent study showed that patients with statin-associated myopathy experienced full resolution of muscle pain on cessation of statin therapy and recurrent muscle pain was noticed on statin rechallenge.11 The American College of Cardiology/American Heart Association/National Heart, Lung, and Blood Institute have summarised the current understanding of the appropriate use and safety of statins (table 3).12

and Blood Institute clinical recommendations for statin myopathy12
Amiodarone is a class III antiarrhythmic with a half-life of 40–50 days. The terminal elimination half-life of amiodarone after long-term oral treatment is approximately 40 days or longer. It is metabolised by the enzyme cytochrome P450 3A4.13 Statins such as atorvastatin, lovastatin and simvastatin are metabolised via a cytochrome P450 3A-dependent pathway while fluvastatin, pravastatin and rosuvastatin are metabolised via cytochrome P450 3A-independent pathways.5 As simvastatin and amiodarone are metabolised by the same isoenzyme, in the present case, the concomitant use of these drugs may have resulted in competition, resulting in excess of free plasma statin and thereby causing myotoxicity. However, there are limited publications on this interaction and the exact mechanism has not
been established.
In the ongoing Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) trial, myopathy was observed in 6% of patients receiving simvastatin 80 mg/day in combination with amiodarone. There is a formal contraindication to the use of simvastatin
80 mg/day with amiodarone in the summary of product characteristics following early safety results from the above study. It was therefore recommended that the dose of simvastatin should not exceed
20 mg/day in patients concomitantly treated with amiodarone. Our patient was taking four times the dose recommended by the manufacturer.14 Alisheikh-Ali and Karas15 studied the percentage of statin-associated adverse event reports with concurrent use of amiodarone. The authors analysed muscle, liver, pancreas and bone marrow systems and found that simvastatin-related adverse reports with concomitant amiodarone use were 1%. The adverse event reports with atorvastatin and pravastatin use were 0.7% and 0.4%, respectively, when combined with amiodarone. About 77% of these adverse event reports were due to muscle toxicity.15 Amiodarone can also cause hypothyroidism, which may be associated with myopathy. In this case the patient’s marginal rise in thyroid stimulating hormone may not explain
his symptoms.
Physicians should be aware of the risk of myopathy when using statins especially when co-prescribed with other drugs that are metabolised by the same isoenzyme, cytochrome P450 3A4. The prescribing physician should consider the co-morbid risk factors, including polypharmacy, and initiate statin at a lower dose in the elderly. Patients should be given adequate information concerning symptoms of myopathy and be advised to seek help promptly if they develop such symptoms.
Conflict of interest
None declared.
Key messages
- Rhabdomyolysis and acute renal failure are potentially serious complications of statin therapy
- Drug interactions resulting in myopathy should be considered
when prescribing statins
References
- Chouhan UM, Chakrabarti S, Millward LJ. Simvastatin interaction with clarithromycin and amiodarone causing myositis. Ann Pharmacother 2005;39:1760–1.
- Ricaurte B, Guirguis A, Taylor HC, Zabriskie D. Simvastatin-amiodarone interaction resulting in rhabdomyolysis, azotemia, and possible hepatotoxicity. Ann Pharmacother 2006;40:753–7.
- Wratchford P, Ponte CD. High-dose simvastatin and rhabdomyolysis. Am J Health Syst Pharm 2003;60:698–700.
- Roten L, Schoenenberger RA, Krahenbuhl S, Schlienger R. Rhabdomyolysis in association with simvastatin and amiodarone. Ann Pharmacother 2004;38:978–81.
- Jamal SM, Eisenberg MJ, Christopoulos S. Rhabdomyolysis associated with hydroxymethylglutaryl-Coenzyme A reductase inhibitors. Am Heart J 2004;147:956–65.
- Bellosta S, Paoletti R, Corsini A. Safety of statins. Focus on clinical pharmacokinetics and drug interactions. Circulation 2004;109(23 suppl):50–7.
- Schmitz G, Drobnik W. Pharmocogenomics and pharmacogenetics of cholesterol-lowering therapy. Clin Chem Lab Med 2003;41:581–9.
- Huerta-Alardin A, Varon J, Marik P. Bench to bedside review: rhabdomyolysis – an overview for clinicians. Crit Care 2005;9:158–69.
- Graham DJ, Staffa JA, Shatin D et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004;292:2582–8.
- Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int 1996;49:314–6.
- Hansen KE, Hildebrand JP, Ferguson EE, Stein JH. Outcomes in 45 patients with statin-associated myopathy. Arch Intern Med 2005;165:2671–6.
- Vasudevan A, Hamirani Y, Jones P. Safety of statins: effects on muscle and the liver. Cleve Clin J Med 2005;72:990–1001.
- Ohyama K, Nakajima M, Suzuki M, Shimada N, Yamazaki H, Yokoi T. Inhibitory effects of amiodarone and its N-deethylated metabolite on human cytochrome P450 activities: prediction of in vivo drug interactions. Br J Clin Pharmacol 2000;49:244–53.
- Physicians’ Desk Reference, 59th ed. Montvale, NJ: Thompson PDR, 2005;2181.
- Alsheikh-Ali A, Karas R. Adverse events with concomitant amiodarone and statin therapy. Prev Cardiol 2005;8(2):95-7.
