The heart beats 120,000 times a day pumping 7,000 litres. Ischaemia or inflammation decimate this workload causing end organ dysfunction. New National Institute for Health and Care Excellence (NICE) guidelines for acute heart failure (AHF) acknowledge very high early mortality. Of 67,000 acute admissions, 11% die in hospital, 50% are readmitted and 33% are dead within 12 months.1,2 When cardiogenic shock ensues, prognosis is poor.3-5 Most patients are elderly but several thousand deaths occur in those under 65 years. Many are salvageable using advanced resuscitation techniques. When the prospectively randomised-controlled trial (RCT) IABP-SHOCK II trial (Intra-Aortic Balloon Pump in Cardiogenic Shock II) revealed the intra-aortic balloon pump (IABP) as ineffective, a New England Journal of Medicine (2012) editorial stated “we must move forward understanding that a condition with 40% mortality at 30 days remains unacceptable”.3,4 So do the guidelines help with this?

NICE’s medical therapy is excellent, until the end game, but drugs alone have limitations.5 Inotropes worsen ischaemia, while vasopressors elevate afterload. Injured myocardium needs rest to promote recovery, not a flogging.6 Consider two real patients. A 21-year-old female with ion-channelopathy is admitted to a tertiary care centre. After 75 DC shocks and cardiac massage she is in shock. She needs extracorporeal membrane oxygenation (ECMO) circulatory support but cannot be transferred.6 She dies. A 56-year-old male with ischaemic cardiomyopathy suffers acute on chronic heart failure. Renal impairment and pulmonary hypertension preclude transplantation. Though an ideal candidate for a long-term left ventricular assist device (LVAD) he dies.6 In the US, this mode of avoidable death is designated ‘failure to rescue’ through inadequate care.7
Rotary blood pumps are appropriately simple for surgeons. They are less complicated than cardiac resynchronisation or implantable defibrillators. Unlike the IABP they restore flow to physiological levels and unload the injured ventricle. Supported by laboratory research in a non-transplant centre, we first achieved LVAD ‘bridge to recovery’ for acute myocarditis in 1996.8 This young patient is still doing well. With the World’s first permanent implant of a rotary LVAD (2000) we achieved 7.5 years of good quality life in end stage dilated cardiomyopathy (15% of overall lifetime).8,9 After many successes, charitable funding expired. Now we have nothing to offer the sickest patients.
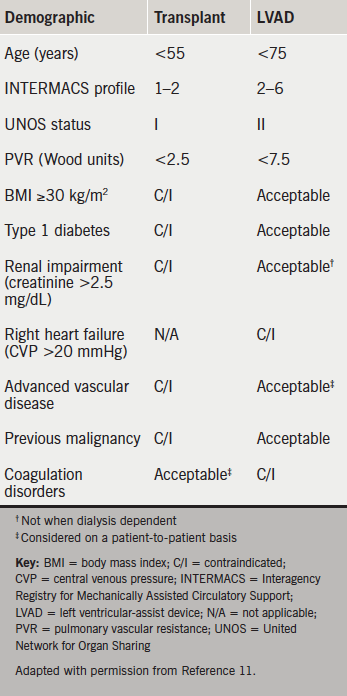
NICE guidelines state “people with potentially reversible severe AHF or potential transplant candidates should be discussed with a centre which provides mechanical circulatory support”.1 This is problematic. Five designated centres perform 100–120 transplants per year with funding for a small number of LVADs.10 They cannot triage all deteriorating patients. Many are too sick to transfer. The National Specialist Commissioning Group fund long-term LVADs only for bridge to transplantation (figure 1). Consequently, devices costing £120,000 are discarded in weeks or months when a donor becomes available. Designed for ‘lifetime’ use, implantable LVAD survival approaches that of transplantation with excellent symptomatic relief (table 1).1-13 The longest exceeds eight years. The oldest patients are nonagenarians.
Unhelpful categorisation
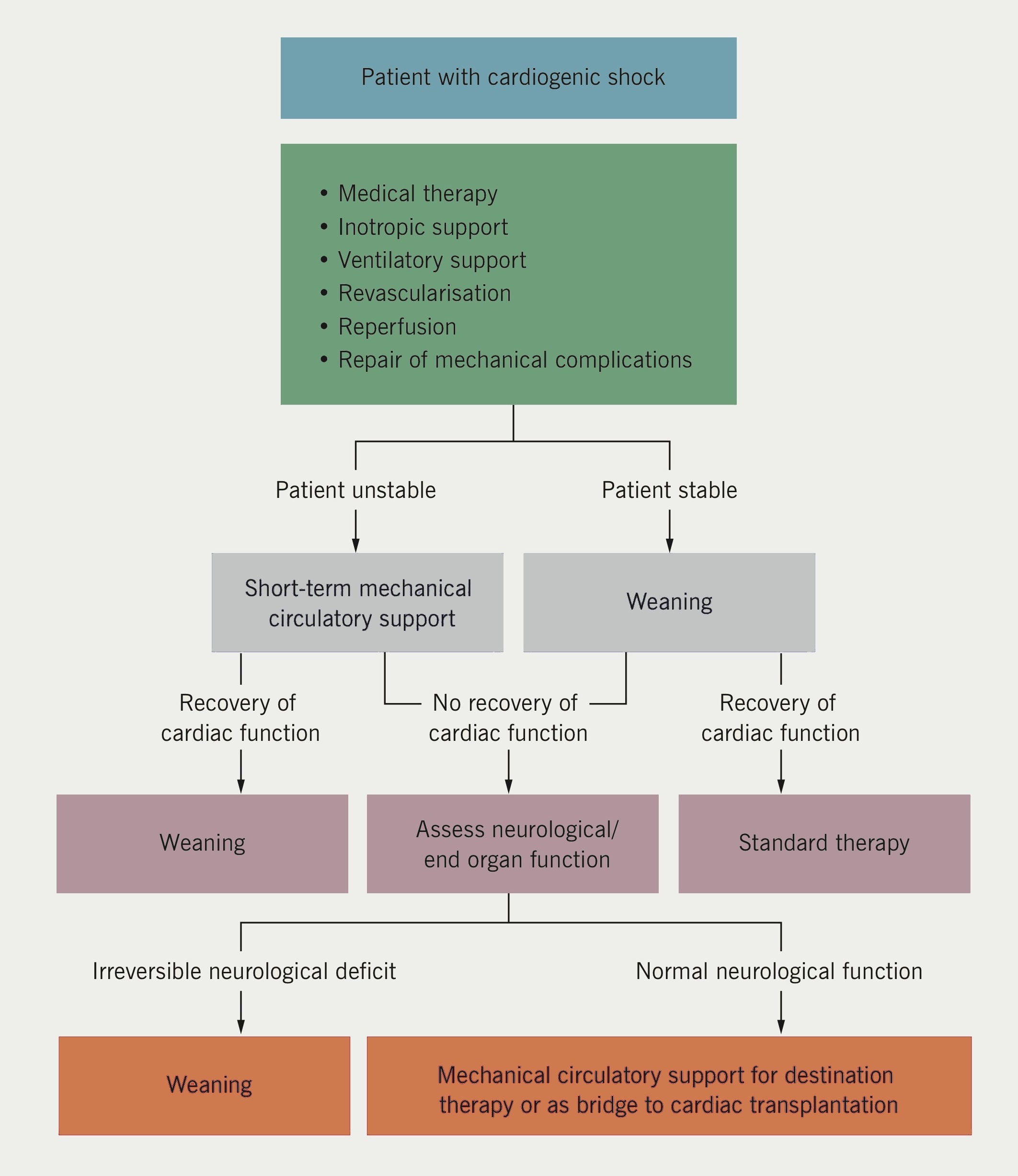
An expert Society of Thoracic Surgeons (US) and Food and Drugs Administration ‘Think Tank’ on circulatory support (2012) deemed categorisation into ‘bridge to transplant’, ‘bridge to recovery’ or ‘lifetime’ use as unhelpful.14 Heart failure is unpredictable.15 The objectives are to relieve symptoms and extend life with the techniques available.16 European Society of Cardiology guidelines specify the use of LVADs and ECMO in AHF and during myocardial revascularisation (figure 2).17,18 The American College of Cardiology and American Heart Association also provide circulatory support algorithms.19 NICE Interventional Procedures Panel (2006) published supportive guidance for temporary LVAD use, but funding was not forthcoming for non-transplant centres.20 Had inexpensive LVADs and ECMO been provided to tertiary centres, several thousand lives could have been saved.21
This time, NICE pronounced on LVAD use based solely upon the review question “for people with AHF is IABP counter pulsation more clinically/cost effective compared to LVADs, medical care alone or each other?”1 Three RCTs showed no advantage of IABP above drugs for shock.3 Three RCTs showed no advantage for two very short-term (2–3 days) percutaneous LVADs over IABP.6 Not included were effective longer-term (weeks or months) extracorporeal LVADs and ECMO, which for ethical reasons cannot be randomised in dying patients. Between 50% and 75% of shock patients survive with a pump.6 Finally, one RCT showed better survival with LVAD over medical management. Deemed ‘low quality evidence’ by NICE, this was the pivotal Randomised Evaluation of Mechanical Assistance in the Treatment of Congestive Heart failure (REMATCH) trial widely considered the most remarkable endeavour in the history of RCTs.22,23 REMATCH launched LVADs as a transplant alternative.11-13
NICE did not acknowledge the inappropriateness of RCTs in dying patients, therefore, the role of circulatory support remains poorly defined. Even the research recommendations make no mention of LVADs.1 The General Medical Council’s guidelines for end-of-life care are relevant in AHF.24 They state “you should not withhold treatment if doing so would involve significant risk to the patient and the only justification is resource constraints”. Duty of candour would suggest that the chances for survival with circulatory support should be discussed with the patient or their relatives, whether or not the devices are immediately available in their hospital. The onus is on the system to provide the means for survival irrespective of geography. Given the impressive survival rates, ECMO and LVADs must not be limited to transplant centres, as European guidelines indicate.25 Every cardiac surgery centre must have temporary circulatory support technology available. In turn, at least 1,000 systolic heart failure patients annually could benefit from an implantable long-term LVAD. This does not need a Medical Innovations Bill. Lifetime LVADs have been funded by US Medicare and Medicaid since 2003. One thousand times £150,000 (LVAD plus hospital costs) is £150 million, and this is the problem. A week after guideline publication, the BBC’s prime time One Show (8 October 2014) raised public expectations by featuring LVADs as the miracle cure for severe heart failure. But the long and short of it is that palliative care and early death are much cheaper.
Conflict of interest
SW was the ‘co-opted’ surgical representative on the NICE Acute Heart Failure Guidelines panel and was invited to attend two meetings. He was overseas representative on the Society of Thoracic Surgeons/Food and Drugs Administration ‘Think Tank’ on long-term mechanical circulatory support. He is co-founder and Medical Director of Calon Cardio-technology.
References
1. National Institute for Health and Care Excellence. Acute heart failure: diagnosing and managing acute heart failure in adults. CG187. London: NICE, 2014. Available from: http://www.nice_org.uk/guidance/cg187
2. Cleland J, Dargie H, Hardman S, McDonagh T, Mitchell P. National heart failure audit. April 2012–March 2013. London: National Institute for Cardiovascular Outcomes Research (NICOR), 2013. Available from: http://www.ucl.ac.uk/nicor/audits/heartfailure/documents/annualreports/hfannual12-13.pdf
3. Thiele H, Zeymer U, Neumann EJ. Intra-aortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–96. http://dx.doi.org/10.1056/NEJMoa1208410
4. O’Connor CM, Rogers JG. Evidence for overturning the guidelines in cardiogenic shock. N Engl J Med 2012;367:1349–50. http://dx.doi.org/10.1056/NEJMe1209601
5. Westaby S, Kharbanda R, Banning AP. Cardiogenic shock in ACS. Part 1: prediction, presentation and medical therapy. Nat Rev Cardiol 2012;9:158–71. http://dx.doi.org/10.1038/nrcardio.2011.194
6. Westaby S, Anastasiadis K, Weiselthaler GM. Cardiogenic shock in ACS. Part 2: role of mechanical circulatory support. Nat Rev Cardiol 2012;9:195–208. http://dx.doi.org/10.1038/nrcardio.2011.205
7. Ahmed EO, Butler R, Novick RJ. Failure to rescue as a measure of quality of care in a cardiac surgery recovery unit: a five year study. Ann Thorac Surg 2014;97:147–52. http://dx.doi.org/10.1016/j.athoracsur.2013.07.097
8. Westaby S, Katsumata T, Piggot D et al. Mechanical bridge to recovery in fulminant myocarditis. Ann Thorac Surg 2000;70:278–82. http://dx.doi.org/10.1016/S0003-4975(00)01450-8
9. Westaby S, Frazier OH, Banning A. Six years of continuous mechanical circulatory support. N Engl J Med 2006;355:325–7. http://dx.doi.org/10.1056/NEJMc060715
10. MacGowan GA, Parry G, Schueler S, Hassan A. The decline in heart transplantation in the UK. BMJ 2011;342:d2483. http://dx.doi.org/10.1136/bmj.d2483
11. Westaby S. Cardiac transplant or rotary blood pump: contemporary evidence. J Thorac Cardiovasc Surg 2013;145:24–31. http://dx.doi.org/10.1016/j.jtcvs.2012.08.048
12. Kirklin JK, Naftel DC, Pagani FD et al. Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation. J Thorac Cardiovasc Surg 2012;144:584–603. http://dx.doi.org/10.1016/j.jtcvs.2012.05.044
13. Shumway SJ. Heart transplantation versus long term mechanical assist devices: clinical equipoise? Eur J Cardiothorac Surg 2013;44:195–7. http://dx.doi.org/10.1093/ejcts/ezt210
14. Acker MA, Pagani FD, Gattis Stough W et al. Statement regarding the pre and post market assessment of durable, implantable ventricular assist devices in the United States. Ann Thorac Surg 2012;94:2147–58. http://dx.doi.org/10.1016/j.athoracsur.2012.09.040
15. Goodlin SJ. Palliative care in congestive heart failure. J Am Coll Cardiol 2009;54:386–96. http://dx.doi.org/10.1016/j.jacc.2009.02.078
16. Westaby S, Deng M. Continuous flow blood pumps: the new gold standard for advanced heart failure. Eur J Cardiothorac Surg 2013;44:4–8. http://dx.doi.org/10.1093/ejcts/ezt248
17. McMurray JJV, Adamopoulos S, Anker SD et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J 2012;33:1787–847. http://dx.doi.org/10.1093/eurheartj/ehs104
18. Windecker S, Kolh P, Alfonso F et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–619. http://dx.doi.org/10.1093/eurheartj/ehu278
19. Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:147–239. http://dx.doi.org/10.1016/j.jacc.2013.05.019
20. National Institute for Health and Care Excellence. Interventional Procedure Guidance 177. Short-term circulatory support with the left ventricular assist devices as a bridge to cardiac transplantation or recovery. London: NICE, June 2006. Available from: http://www.nice.org.uk/guidance/ipg177
21. Westaby S, Taggart D. Inappropriate restrictions on life saving technology. Heart 2012;98:1117–19. http://dx.doi.org/10.1136/heartjnl-2011-301365
22. Rose EA, Gelijns AC, Moskowitz AJ et al. Long term mechanical left ventricular assistance for end stage heart failure. N Engl J Med 2001;345:1435–43. http://dx.doi.org/10.1056/NEJMoa012175
23. Leitz K, Miller LW. Will left ventricular assist device therapy replace heart transplantation in the foreseeable future? Curr Op Cardiol 2005;20:132–7.
24. General Medical Council. Treatment and care towards the end of life: good practice in decision making. Manchester: GMC, 20 May 2010. Available from: http://www.gmc-uk.org/guidance/ethical_guidance/end_of_life_care.asp
25. Westaby S. Lifetime circulatory support must not be limited to transplant centres. Heart Fail Clin 2007;3:369–75. http://dx.doi.org/10.1016/j.hfc.2007.05.008
