We present a review of transcatheter aortic valve implantation (TAVI) in the presence of a mechanical mitral valve. We conclude that in patients with a prior mechanical prosthesis, TAVI is feasible and can be carried out without complication. Based on proof of feasibility, evidence to date would suggest that patients with mechanical prostheses be actively considered for TAVI going forward.
Introduction
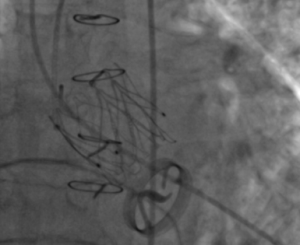
Transcatheter aortic valve implantation (TAVI) has become standard of care for patients with severe aortic stenosis at prohibitive operative risk for surgical aortic valve replacement (SAVR).1 The first randomised-controlled trial of TAVI stipulated the presence of a mitral valve prosthesis as an exclusion criterion for enrolment in the trial.2 The main reason was concern that dysfunction of the mitral valve prosthesis might arise during TAVI valve deployment.3 Further concerns were that the presence of a rigid mitral prosthesis would inhibit complete valve deployment or would result in the valve slipping; a ‘watermelon seed’ effect.4 Despite this, a number of authors have published successful cases of TAVI in the presence of mechanical valve prostheses.
Methods
In order to determine the performance of TAVI in the presence of a mechanical mitral prosthesis we performed a systematic review of all published cases. Specifically, we sought to evaluate the clinical efficacy of TAVI in patients who had previously undergone mechanical mitral valve replacement. The review was undertaken in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The primary outcome of interest was mortality at 30 days. Secondary outcomes included dysfunction of the mechanical prosthesis, TAVI valve embolisation, endocarditis, under-expansion of the TAVI valve and paravalvular leak, procedural success, and conversion to SAVR. Outcomes are reported in accordance with the Valve Academic Research Consortium (VARC) 2 consensus document.5
An online search of PubMed was performed on 30 August 2014. Search terms used included permutations of percutaneous, transcutaneous, transcatheter, transarterial, transfemoral, transapical, TAVI, transcatheter aortic valve replacement (TAVR), mechanical mitral prosthesis. Additional studies were identified using references cited within appropriate articles. Data were extracted by two independent authors (KOS, EH) and discrepancies were resolved by discussion.
Results
We identified 397 studies of which 16 were selected for full review. Of these, 11 were isolated case reports, and five were case series reporting two or more cases. A total of 35 cases were identified. Fifteen cases describe Medtronic CoreValve® implantation, 15 Edward Sapien®, three Sapien XT, one Medtronic Evolut™, and one JenaValve™ (figure 1. One case describes CoreValve® implantation with an interval subsequent implantation of an Edward Sapien®. Of these, eight authors report transfemoral cases (49%, n=17), outlined in table 1. Ten authors describe a transapical approach (40%, n=14), outlined in table 2. One author outlines cases performed via a transaortic approach (11%, n=4).
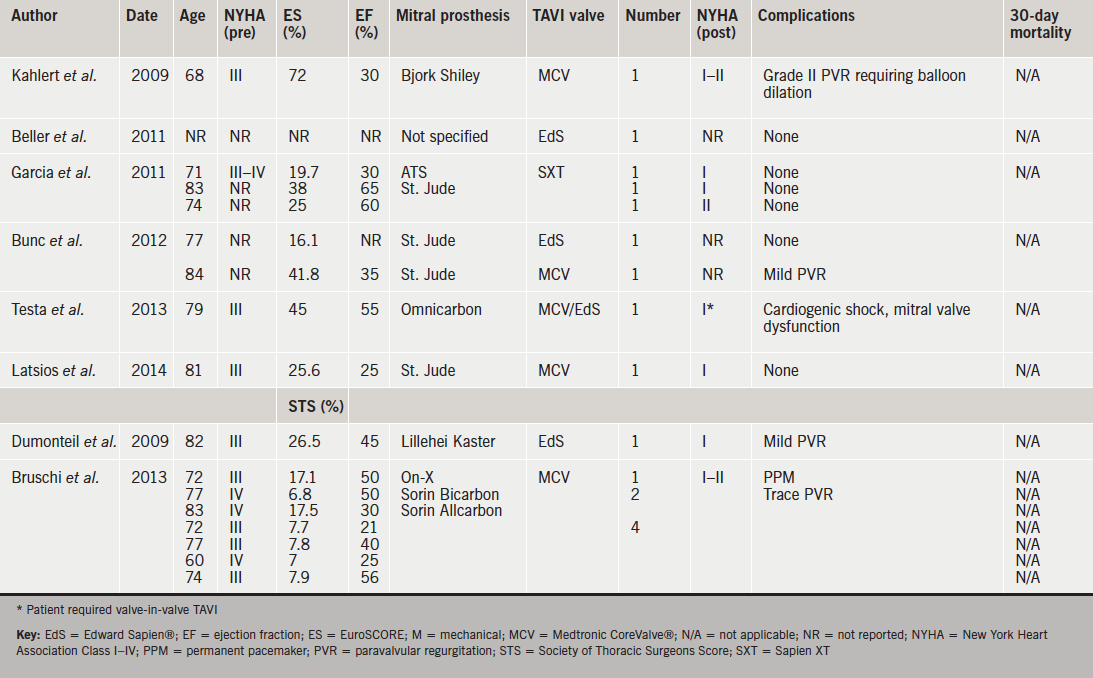
The 30-day mortality rate post-TAVI was 5.7% (n=2). One patient was a 37-year-old woman with severe familial cholesterolaemia who had undergone a portocaval shunt in childhood and implantation of an apico-descending conduit four years prior, due to severe aortic calcification and a porcelain aorta. She underwent a subsequent mechanical mitral valve replacement. Her ejection fraction was 10%, EuroSCORE 85% and Society of Thoracic Surgeons predicted risk of mortality (STS PROM) was 75%. In this case, the valve was successfully deployed via the apex of the heart but severe left ventricular failure necessitated extracorporeal membrane oxygenation and the patient developed septicaemia and died on day 5.6 Long-term follow-up of reported cases was limited. However, one mortality eight months post-procedure was reported in a patient with aortic valve endocarditis requiring re-operation who died of multi-organ failure post-procedure.6
Late transcatheter heart valve embolisation has been identified in two reported cases.7 The first, a 75-year-old female with a prior mechanical prosthesis who, subsequent to transapical TAVI, presented with acute cardiac failure and an embolised Edward Sapien® prosthesis 21 days following implantation with a tilted valve and severe aortic regurgitation. The valve was retrieved at SAVR, however, she suffered a stroke and died seven days following surgery, so is, therefore, considered a 30-day mortality.8 The second was an 82-year-old female who re-presented 14 days following transapical TAVI with recurrent cardiac failure symptoms. Again, in this case, the TAVI prosthesis, an Edward Sapien® valve was tilted, resulting in severe aortic regurgitation. She underwent SAVR and aortic root replacement and was discharged successfully on day 14.9
Procedural success is reported in 97% of cases. There was no conversion to SAVR reported at the time of initial implantation. Testa et al. report implantation of a CoreValve® prosthesis in a patient with an Omnicarbon single-leaflet mechanical mitral prosthesis via the transfemoral approach.10 Authors describe the occurrence of cardiogenic shock, secondary to impairment of the mechanical prosthesis, due to it being situated too inferiorly. Suspecting the aetiology of the complication straightaway, they responded rapidly by first attempting to snare the CoreValve® and pull it back, then releasing it from the annulus and moving it into the ascending aorta. The patient recovered, and two months later underwent a transfemoral Edward Sapien® implantation by crossing the CoreValve®, which remained in the aortic position. She was discharged with significant symptomatic improvement on day 6.10
Procedural complications were reported in 6% (n=2). In one case of transfemoral CoreValve® implantation in a patient with a Bjork Shiley mitral prosthesis, grade two paravalvular regurgitation following valve deployment warranted balloon dilation.11 The second case describes transient mechanical prosthesis dysfunction resulting from the transapical delivery system touching a leaflet of the mechanical prosthesis. Upon immediate retraction the issue resolved and the subsequent implantation was uneventful.6 Interestingly, despite frequently cited concerns regarding the ‘watermelon seed’ effect, there have been no reported cases of this occurring to date. Furthermore, there were no reported cases of valve deformation. Post-procedural complications are reported in 11.4% (n=4) of cases. Specifically, one instance of pleural effusion following transapical TAVI, which required drainage. Also one patient in whom a permanent pacemaker was required.12,13 Paravalvular regurgitation, described as trace-mild, was reported in three cases.12,14,15
Discussion
Despite initial concerns, feasibility of transapical, transaortic and transfemoral TAVI in the presence of a mechanical prosthesis has been demonstrated. Experience to date, however, is limited and this patient cohort represents a relatively small proportion of the overall group eligible for TAVI. Considering our systematic review predominantly identified case reports, it is difficult to draw any firm conclusions regarding complication rates and 30-day mortality compared with larger patient cohorts. Furthermore, there is a significant risk of publication bias with authors more likely to publish successful cases of implantation than incidences of failure. This creates a likelihood of underestimation of the potential dangers involved. Nonetheless, the complications reported in cases published are well accepted; pacemaker insertion, endocarditis and mild paravalvular leak.2 What makes this group unique is the propensity for mechanical valve dysfunction, either intra- or post-procedurally. While two authors report this, it is likely that there is under-reporting of its incidence.6,10 It is difficult to recommend an optimum vascular approach based on the limited experience to date. Some would argue that the transaortic route offers the benefits of the control obtained via transapical TAVI without the additional risk of delivering the delivery sheath ‘past’ the mitral prosthesis in the left ventricle.16 This belief is unsubstantiated by evidence to date and is, therefore, speculative at present. In the absence of solid evidence, it is still reasonable that the least invasive approach should be adopted. Furthermore, it is possible that prosthesis choice is an important factor in this setting, one case report describes the use of the JenaValve, whereby a potential advantage of the prosthesis is the fact that there is a limit to the lower extent of valve deployment in the left ventricle due to the leaflet clipping mechanism built into the valve design.13 Again, while logical, there is insufficient evidence to date to conclusively recommend this.
One concern is that of late valve embolisation, as identified in two reported cases. While other issues likely to predispose to late embolisation exist, such as valve under sizing, under expansion of an appropriately sized valve due to aortic root calcification, asymmetric aortic root calcification, basal septal bulging and low valve implantation, the presence of a mechanical valve is likely to precipitate superior displacement of the TAVI prosthesis and remains an associated risk.7 Indeed, one of the reported cases at SAVR noted non-circularity of the aortic annulus in the region of the non-coronary sinus, the specific area in which the TAVI prosthesis had slipped into the ventricle.9
This study has a number of limitations. Case reports comprise the majority of the reported patients; therefore, there is likely bias towards under-reporting of adverse events and complications. This represents a small subpopulation of TAVI patients and is likely to be a scenario encountered quite rarely by each reporting author. Nonetheless, the review we have carried out does have value to those clinicians being faced with this challenge.
Conclusion
We demonstrate that TAVI, in patients with a prior mechanical prosthesis, is not only feasible but can be carried out without complication. Meticulous procedural planning is a necessity. Based on proof of feasibility, evidence to date would suggest that patients with mechanical prostheses be actively considered for TAVI going forward. Further studies are required to identify the potential benefits of certain prostheses in this setting.
Conflict of interest
None declared.
Key messages
- Presence of a mechanical mitral valve was an exclusion criterion of the PARTNER (Placement of Aortic Transcatheter Valves) trial, however, to date 35 cases have been described in the literature
- TAVI deployment in the presence of a mechanical mitral prosthesis is feasible, but should be approached with caution; incidences of mitral valve ‘locking’ have been reported
- Strategies for success include pre-procedural study of the existing prosthesis, close examination of the aorto-mitral area, and shape of balloon at valvuloplasty, careful prosthesis selection and slow valve deployment
References
1. Holmes DR Jr, Mack MJ, Kaul S et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012;59:1200–54. http://dx.doi.org/10.1016/j.jacc.2012.01.001
2. Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607. http://dx.doi.org/10.1056/NEJMoa1008232
3. Chao VT, Chiam PT, Tan SY. Transcatheter aortic valve implantation with preexisting mechanical mitral prosthesis – use of CT angiography. J Invasive Cardiol 2010;22:339–40. Available from: http://www.invasivecardiology.com/articles/Transcatheter-Aortic-Valve-Implantation-with-Preexisting-Mechanical-Mitral-Prosthesis-—-Use
4. Garcia E, Albarran A, Heredia-Mantrana J et al. [Transcatheter aortic valve implantation in patients with a mechanical mitral valve]. Rev Esp Cardiol 2011;64:1052–5. http://dx.doi.org/10.1016/j.recesp.2011.02.022
5. Kappetein AP, Head SJ, Genereux P et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surgery 2013;145:6–23. http://dx.doi.org/10.1016/j.jtcvs.2012.09.002
6. Drews T, Pasic M, Buz S et al. Transapical aortic valve implantation after previous mitral valve surgery. J Thorac Cardiovasc Surg 2011;142:84–8. http://dx.doi.org/10.1016/j.jtcvs.2010.08.034
7. Mylotte D, Andalib A, Theriault-Lauzier P et al. Transcatheter heart valve failure: a systematic review. Eur Heart J 2014;published online. http://dx.doi.org/10.1093/eurheartj/ehu388
8. Maroto LC, Rodriguez JE, Cobiella J, Silva J. Delayed dislocation of a transapically implanted aortic bioprosthesis. Eur J Cardiothorac Surg 2009;36:935–7. http://dx.doi.org/10.1016/j.ejcts.2009.03.072
9. Baumbach H, Hill S, Hansen M, Franke UF. Severe aortic insufficiency after transapical aortic valve implantation. Ann Thorac Surg 2011;92:728–9. http://dx.doi.org/10.1016/j.athoracsur.2011.02.047
10. Testa L, Gelpi G, Bedogni F. Transcatheter aortic valve implantation in a patient with mechanical mitral prosthesis: a lesson learned from an intraventricular clash. Catheter Cardiovasc Interv 2013;82:E621–E625. http://dx.doi.org/10.1002/ccd.24948
11. Kahlert P, Eggebrecht H, Thielmann M et al. Transfemoral aortic valve implantation in a patient with prior mechanical mitral valve replacement. Herz 2009;34:645–7. http://dx.doi.org/10.1007/s00059-009-3295-5
12. Bruschi G, De Marco F, Barosi A et al. Self-expandable transcatheter aortic valve implantation for aortic stenosis after mitral valve surgery. Interact Cardiovasc Thorac Surg 2013;17:90–5. http://dx.doi.org/10.1093/icvts/ivt086
13. O’Sullivan KE, Casserly I, Hurley J. Transapical JenaValve in a patient with mechanical mitral valve prosthesis. Catheter Cardiovasc Interv 2014;published online. http://dx.doi.org/10.1002/ccd.25415
14. Dumonteil N, Marcheix B, Berthoumieu P et al. Transfemoral aortic valve implantation with pre-existent mechanical mitral prosthesis: evidence of feasibility. JACC Cardiovasc Interv 2009;2:897–8. http://dx.doi.org/10.1016/j.jcin.2009.05.023
15. Bunc M, Ambrozic J, Music S et al. Successful Tavi in patients with previous mitral valve replacement. Int J Clin Med 2012;3:603–06. http://dx.doi.org/10.4236/ijcm.2012.37109
16. Bruschi G, De Marco F, Oreglia J et al. Percutaneous implantation of CoreValve aortic prostheses in patients with a mechanical mitral valve. Ann Thorac Surg 2009;88:e50–e52. http://dx.doi.org/10.1016/j.athoracsur.2009.07.028

