In this article we review the latest cardiovascular outcomes trials performed using older diabetes drugs.
Introduction
 The past decade has seen the emergence of several new classes of drugs for the treatment of type 2 diabetes mellitus (T2DM). Despite the increasing use of these agents, metformin and sulfonylureas remain the most commonly prescribed glucose-lowering therapies in T2DM.1 This reflects the National Institute for Health and Care Excellence (NICE) and Scottish Intercollegiate Guidelines Network (SIGN) guidelines, which recommend metformin as first-line treatment, and sulfonylureas as a potential second-line treatment, for patients with T2DM.2,3 Insulin is an important treatment in T2DM for optimisation of osmotic symptoms and glycaemic control. Acarbose is prescribed infrequently in the UK, however, its use remains widespread in Asia. These ‘older’ therapies pre-date the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) requirements for pre-specified cardiovascular (CV) safety trials for new T2DM treatments and were not subject to the same rigorous clinical trial programmes to examine cardiovascular safety. However, several recent cardiovascular outcomes trials have been performed using these drugs.
The past decade has seen the emergence of several new classes of drugs for the treatment of type 2 diabetes mellitus (T2DM). Despite the increasing use of these agents, metformin and sulfonylureas remain the most commonly prescribed glucose-lowering therapies in T2DM.1 This reflects the National Institute for Health and Care Excellence (NICE) and Scottish Intercollegiate Guidelines Network (SIGN) guidelines, which recommend metformin as first-line treatment, and sulfonylureas as a potential second-line treatment, for patients with T2DM.2,3 Insulin is an important treatment in T2DM for optimisation of osmotic symptoms and glycaemic control. Acarbose is prescribed infrequently in the UK, however, its use remains widespread in Asia. These ‘older’ therapies pre-date the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) requirements for pre-specified cardiovascular (CV) safety trials for new T2DM treatments and were not subject to the same rigorous clinical trial programmes to examine cardiovascular safety. However, several recent cardiovascular outcomes trials have been performed using these drugs.
Metformin
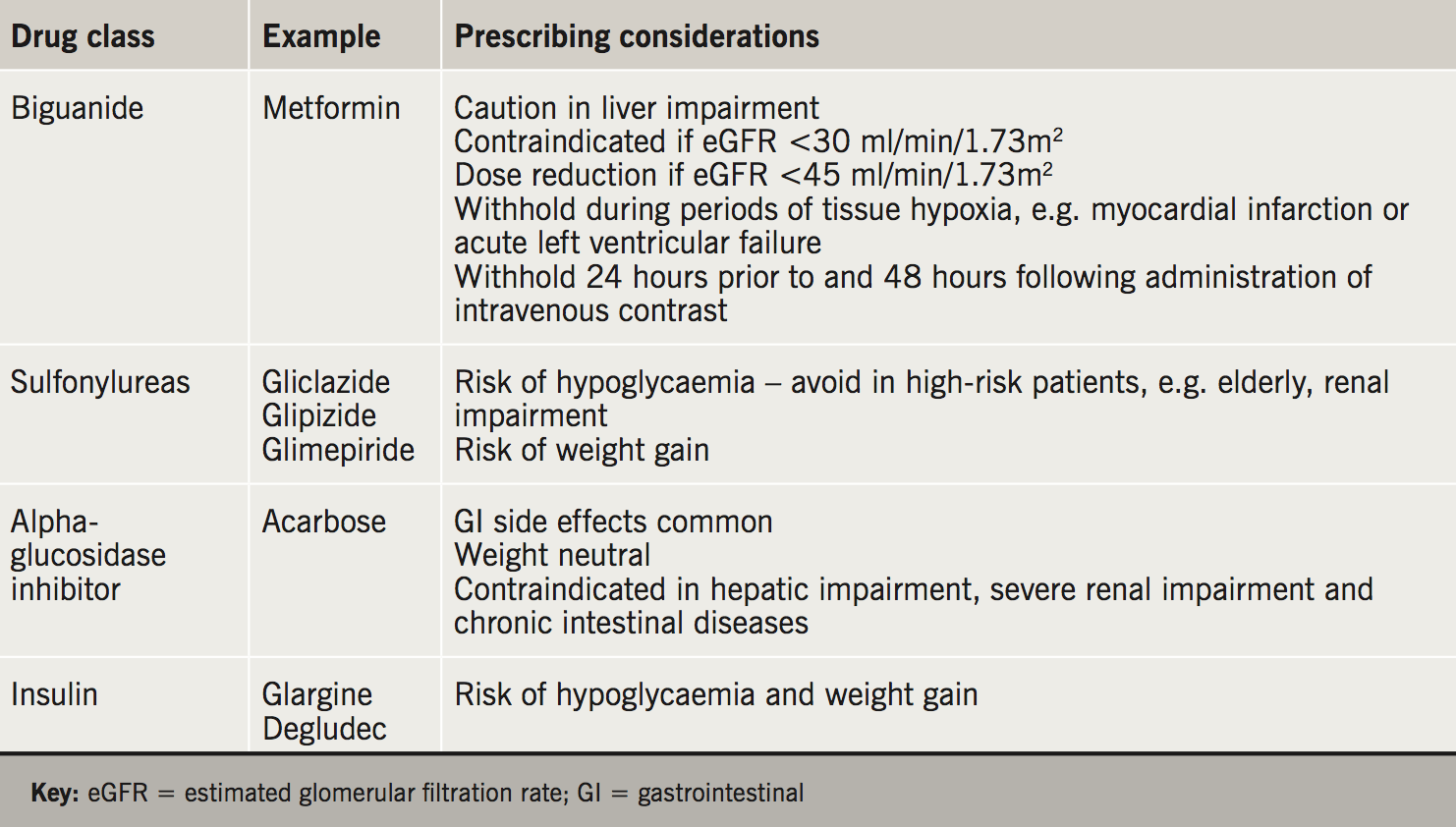
Metformin has a complex mechanism of action, which includes inhibition of hepatic gluconeogenesis, increased peripheral insulin sensitivity and improved peripheral glucose utilisation. It improves glycaemic control and does not typically cause hypoglycaemia or weight gain. Gastrointestinal (GI) upset is the most common side effect and the risk is minimised by gradual dose titration. It is recommended as the first-line therapy for T2DM in the NICE and SIGN guidelines.2,3
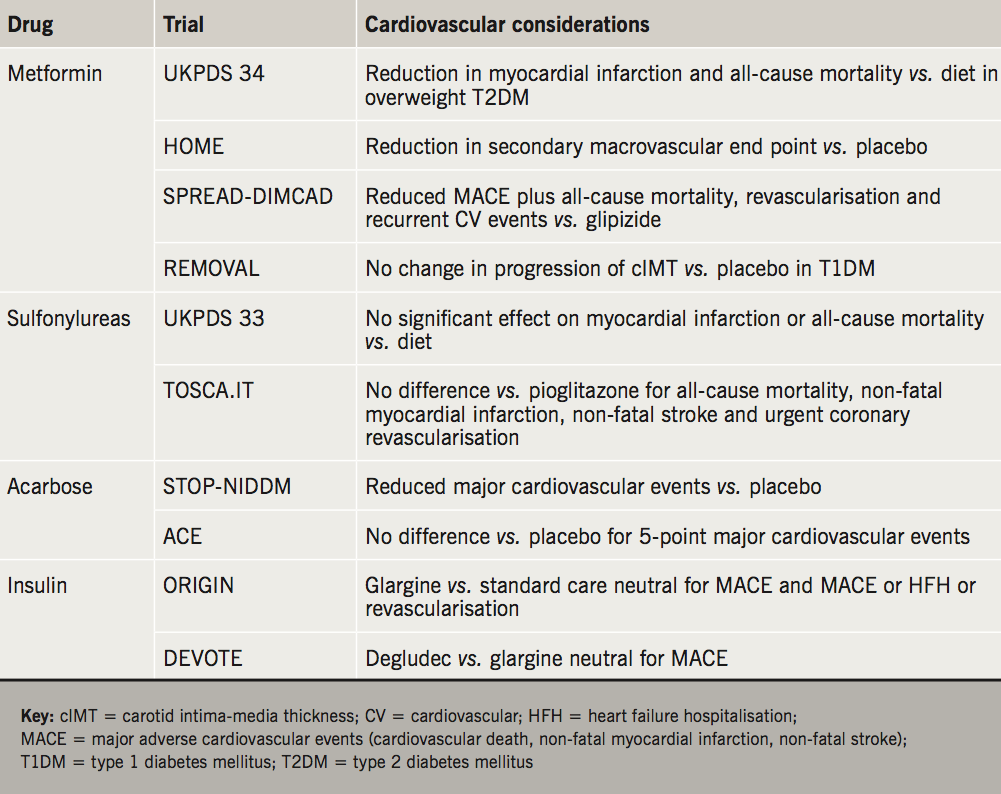
In the United Kingdom Prospective Diabetes Study (UKPDS) a subgroup of 753 overweight patients were randomised to receive intensive treatment with metformin (n=342) or conventional treatment with dietary advice alone (n=411).4 Over the median follow-up period of 10.7 years, metformin was associated with significant relative risk reductions in diabetes-related death (42%), all-cause mortality (36%) and myocardial infarction (39%). Support for a cardiovascular benefit of metformin in T2DM comes from a post-hoc analysis of HOME (Hyperinsulinaemia: the Outcome of Its Metabolic Effects, a Randomized Controlled Trial), which examined the impact of metformin add-on therapy for patients with insulin-treated T2DM, and found a reduction in a composite of non-adjudicated macrovascular events as a secondary outcome.5,6
More recently, SPREAD-DIMCAD (Study on the Prognosis and Effect of Anti-Diabetic Drugs on Type-2 Diabetes Mellitus With Coronary Artery Disease) randomised 304 patients with T2DM and coronary artery disease to receive double-blind metformin (1.5 g daily) or glipizide (30 mg daily) for three years.7 Metformin was associated with a 46% relative risk reduction in the primary composite outcome (recurrent CV events, CV death, all-cause mortality, non-fatal myocardial infarction, non-fatal stroke or arterial revascularisation) compared with the glipizide group (95% confidence interval [CI] 0.3 to 0.9). End points were obtained from routine clinical notes.
REMOVAL
The REMOVAL (REducing With MetfOrmin Vascular Adverse Lesions in Type 1 Diabetes) trial evaluated the impact of metformin on progression of atherosclerosis in patients with type 1 diabetes (T1DM) and increased cardiovascular risk.8 The trial recruited 428 patients with baseline HbA1c 8% from 23 outpatient clinics across Europe, Canada and Australia. Patients were aged 40 years or more, had a minimum diabetes duration of five years, and possessed at least three of 10 pre-specified CV risk factors. Following a three-month run-in period with placebo, patients were randomised to receive metformin 1 g twice daily or matching placebo.
The primary outcome was progression of common carotid artery intima-media thickness (cIMT), a surrogate of atherosclerosis, which has been shown to predict future CV events in the general population. Across the three-year treatment period, metformin had no effect on the progression of mean cIMT compared with placebo. Metformin did demonstrate beneficial effects on secondary end points, with reduced bodyweight (–1.17 kg; 95% CI –1.66 to –0.69) and low-density lipoprotein (LDL)-cholesterol (–0.13 mmol/L; 95% CI –0.24 to –0.03). Metformin was also associated with a significant reduction in mean HbA1c over three years (–0.13%; 95% CI –0.22 to –0.037), however, this was accounted for by a reduction after three months follow-up, which was not sustained thereafter. There was no significant difference in insulin requirement between groups across the follow-up period.
Sulfonylureas
Sulfonylureas augment insulin release from pancreatic beta cells through binding to SUR1 receptors and closure of ATP-K+ channels. They provide effective glucose lowering but have the unwanted side effects of hypoglycaemia and weight gain. They are recommended in NICE and SIGN guidelines as an add-on therapy to metformin if glycaemic targets are not achieved.2,3 SIGN also acknowledges sulfonylureas as first-line therapy if patients are intolerant to metformin or have weight loss and/or osmotic symptoms.3
The UKPDS assigned patients with newly diagnosed T2DM to conventional diet control (n=1,138) or intensive control with sulfonylurea (chlorpropamide, glibenclamide or glipizide) or insulin (n=2,729).9 The intensive group saw a reduction in risk of 12% for any diabetes-related end point. There was a numerical, but not statistically significant, reduction in myocardial infarctions and all-cause mortality in the intensive treatment group. Outcomes within the intensive group did not differ comparing sulfonylurea and insulin-treated subjects. Ten-year follow-up demonstrated persistent benefits in the intensive treatment group, with relative risk reductions in myocardial infarction (15%) and death from any cause (13%) compared with the conventional group.10
TOSCA.IT
TOSCA.IT (Thiazolidinediones Or Sulfonylureas and Cardiovascular Accidents.Intervention Trial) was an investigator-led open-label trial that compared the impact of sulfonylureas and thiazolidinediones on cardiovascular outcomes.11 There were 3,028 patients with T2DM inadequately controlled on metformin monotherapy randomised to receive sulfonylurea (mostly gliclazide and glimepiride) or pioglitazone as add-on therapy. The trial was stopped based on futility after a median follow-up period of 58 months. A primary end point, comprising all-cause mortality, non-fatal myocardial infarction, non-fatal stroke and urgent coronary revascularisation, occurred in 105 patients in the pioglitazone group and 108 patients in the sulfonylurea group (hazard ratio [HR] 0.96; 95% CI 0.74 to 1.26, p=0.79). There are multiple criticisms of this flawed study; the open-label nature of the design could have introduced bias, especially concerning some of the components of the end point, the event rate was lower than predicted, and low doses of drugs were used. As the study was stopped for futility, it does not inform us in any way about the relative safety of pioglitazone or sulfonylureas.
Acarbose
Acarbose reversibly inhibits the alpha-glucosidase enzyme in the intestinal brush border, reducing the breakdown of non-absorbable complex carbohydrates into absorbable monosaccharides.12 It slows carbohydrate absorption and attenuates post-prandial hyperglycaemia. HbA1c lowering of 0.8% compared with placebo was reported in one meta-analysis.13 Acarbose is associated with GI side effects in up to 30% of patients and is used infrequently in the UK for this reason.14
In STOP-NIDDM (Study to Prevent Non-Insulin Dependent Diabetes Mellitus) 1,429 patients with impaired glucose tolerance (IGT) were randomised to receive acarbose 100 mg or matching placebo three times daily.15 There was a 25% reduction in the incidence of diabetes in the acarbose group after one year. A pre-specified secondary end point showed a reduction in major cardiovascular events in the acarbose group.16 However, a Cochrane review recommended that a definitive conclusion should not be drawn from these data given that the trial was not initially powered to assess CV outcomes, and the number of events was small.13
ACE
The recent investigator-initiated ACE (Acarbose Cardiovascular Evaluation) trial hypothesised that acarbose would reduce CV events in patients with IGT and coronary heart disease.17 There were 6,522 patients, recruited from 176 outpatient clinics in China, randomised to receive acarbose 50 mg three times daily or matched placebo for a median follow-up period of five years. The 50 mg dose was selected due to the high rate of study drug discontinuation with 100 mg three times daily in the STOP-NIDDM trial (31% acarbose, 19% placebo), mainly due to dose-dependent GI side effects.
In ACE, the primary outcome, a five-point major adverse cardiovascular events (MACE) composite (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, hospital admission for unstable angina and hospitalisation for heart failure) occurred in 470 patients in the acarbose group and 479 patients in the placebo group (14% vs. 15%; HR 0.98; 95% CI 0.86 to 1.11; p=0.73). Acarbose was associated with an 18% reduction in the risk of new-onset diabetes. GI symptoms were the most common adverse event, leading to dose change or drug discontinuation in 7% of patients receiving acarbose and 5% receiving placebo. There was a high rate of premature study drug discontinuation in both groups (acarbose 49%, placebo 51%) with a median treatment duration of 3.0 years.
Insulin
Insulin remains an important treatment for T2DM inadequately controlled by oral therapies, or where osmotic symptoms and weight loss are predominant symptoms. Insulin use in the UK trebled between years 1991 and 2010, largely attributed to increased use in patients with T2DM.18
ORIGIN (Outcome Reduction With Initial Glargine Intervention) evaluated the impact of open-label insulin glargine compared with standard care on cardiovascular outcomes, and there was no difference observed in either of the two co-primary outcomes: CV death, non-fatal myocardial infarction or non-fatal stroke; and a composite of these events, revascularisation or heart failure hospitalisation.19
DEVOTE
Insulin degludec is a recent addition to the family of analogue basal insulins: it possesses a unique ultra-flat profile, which allows for flexibility in administration timing without deterioration in glycaemic control or increased risk of hypoglycaemia.20 The FDA requested a dedicated cardiovascular outcomes trial with degludec prior to approval in the US following analysis of events in the phase 3 development programme. DEVOTE (A Trial Comparing Cardiovascular Safety of Insulin Degludec Versus Insulin Glargine in Subjects With Type 2 Diabetes at High Risk of Cardiovascular Events) examined the cardiovascular safety of degludec in 7,637 patients with T2DM recruited from 438 sites in 20 countries.21 Patients were randomised in a double-blind, 1:1 fashion, to receive insulin degludec or insulin glargine once-daily added to standard care for a median follow-up period of two years. Baseline characteristics included mean HbA1c 8.4 ± 1.7%, mean duration of diabetes 16.4 years, and established CV disease, chronic kidney disease, or both, in 85.2%. There were 6,409 (83.9%) patients treated with insulin at baseline, including 3,515 (54.8%) receiving a basal-bolus regimen.
The primary outcome, a three-point MACE composite comprising CV death, non-fatal myocardial infarction and non-fatal stroke, occurred in similar numbers of patients in the degludec and glargine groups (8.5 vs. 9.3%; HR 0.91; 95% CI 0.78 to 1.06; p<0.001 for non-inferiority). After two years the mean HbA1c was the same in both groups (7.5 ± 1.2%), although there was a small reduction in fasting plasma glucose levels in the degludec group. Severe hypoglycaemia occurred less frequently in the degludec group compared with the glargine group (4.9 vs. 6.6%; odds ratio 0.73; p<0.001 for superiority), including severe nocturnal hypoglycaemia. There was no difference in adverse events between the two groups.
Discussion
Metformin is considered as first-line therapy for T2DM due to its longstanding record of safety and efficacy, in addition to low cost. Despite the absence of a dedicated randomised, placebo-controlled trial examining CV outcomes, there is evidence from UKPDS to suggest metformin is associated with reductions in cardiovascular outcomes. Previous concerns regarding the safety of metformin in chronic heart failure have been discounted.22 It is, therefore, recommended by the European Society of Cardiology as the first-line therapy for patients with T2DM and concomitant heart failure.23 Along with lowering glucose, proposed mechanisms by which metformin may reduce CV events include improved endothelial function, antioxidant and anti-inflammatory properties.24
While sulfonylureas and insulin were also associated with improvement in CV end points in the UKPDS post-trial monitoring, the overall evidence of CV benefit with sulfonylureas is less conclusive than metformin. It is hypothesised that through the effect on cardiac ATP-K+ channels, sulfonylureas may prevent the protective process of ischaemic preconditioning, which can offset damage caused to cardiac myocytes from significant myocardial ischaemia.25 This factor may contribute to the lesser cardiovascular benefits demonstrated when compared with metformin in several trials, including 10-year UKPDS follow-up data and SPREAD-DIMCAD. Glimepiride is the active comparator to linagliptin in CAROLINA (Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes), a large CV outcomes study involving patients with early T2DM and increased CV risk or established complications.26 The study is expected to complete in 2019.
Acarbose has demonstrated cardiovascular safety and reduced diabetes incidence compared with placebo in patients with impaired glucose tolerance. It is used very infrequently in the UK because of GI side effects, and the studies discussed seem unlikely to change this. At present, there is no evidence that any insulin provides cardiovascular benefit compared with another. Degludec has demonstrated reduced severe hypoglycaemia, especially severe nocturnal hypoglycaemia, compared with glargine, and may provide a therapeutic option in patients with T1DM and T2DM and problematic hypoglycaemia.
Key messages
- Metformin is the most commonly prescribed glucose-lowering agent used in type 2 diabetes mellitus (T2DM) with evidence to suggest cardiovascular benefit seen in the UKPDS trial and a post-hoc analysis of the HOME trial
- The cardiovascular benefit of sulfonylureas and insulin is less well established but they remain important adjunctive treatments for many patients with T2DM
- In a large double-blinded cardiovascular outcomes trial, insulin degludec was non-inferior to insulin glargine in reducing the primary composite end point of cardiovascular death, non-fatal myocardial infarction and non-fatal stroke in patients with T2DM and established cardiovascular disease
- Acarbose has demonstrated cardiovascular safety and reduced the incidence of newly diagnosed T2DM in patients with impaired glucose tolerance in two randomised, placebo-controlled trials, however, its use remains uncommon in the UK due to gastrointestinal side effects
Conflict of interest
EJ: none declared. GM was a principal investigator for the REMOVAL trial and has received payments for advisory boards from Novo Nordisk and Sanofi. MF was an events adjudicator for the ACE trial and has received payments for lectures and advisory boards from Novo Nordisk and Sanofi.
Editors’ note
This article is the fifth in the series ‘Drugs for diabetes’. Previous articles have covered dipeptidyl peptidase-4 (DPP-4) inhibitors (doi:10.5837/bjc.2017.001), SGLT2 inhibitors (doi:10.5837/bjc.2017.010), glitazones (thiazolidinediones) (doi:10.5837/bjc.2017.018) and glucagon-like peptide-1 (GLP-1) receptor antagonists (doi:10.5837/bjc.2017.030). The last article in the series will cover glucose-lowering drugs for patients with cardiac disease (doi:10.5837/bjc.2018.016).
References
1. NHS Digital. Prescribing for Diabetes, England – 2006/07 to 2016/17. Available from: https://digital.nhs.uk/catalogue/PUB30043 [accessed 25 October 2017].
2. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE Guideline. London: NICE, 2015. Available from: https://www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-in-adults-management-1837338615493 [accessed 17 September 2016].
3. Scottish Intercollegiate Guidelines Network (SIGN). Pharmacological management of glycaemic control in people with type 2 diabetes (Guideline 154). Edinburgh: SIGN, 2017. Available from: http://www.sign.ac.uk/assets/sign154.pdf [accessed 3 January 2018].
4. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–65. https://doi.org/10.1016/S0140-6736(98)07037-8
5. Wulffelé MG, Kooy A, Lehert P et al. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care 2002;25:2133–40. https://doi.org/10.2337/diacare.25.12.2133
6. Kooy A, de Jager J, Lehert P et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009;169:616. https://doi.org/10.1001/archinternmed.2009.20
7. Hong J, Zhang Y, Lai S et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013;36:1304–11. https://doi.org/10.2337/dc12-0719
8. Petrie JR, Chaturvedi N, Ford I et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2017;5:597–609. https://doi.org/10.1016/S2213-8587(17)30194-8
9. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–53. https://doi.org/10.1016/S0140-6736(98)07019-6
10. Holman RR, Paul SK, Bethel MA, Matthews DR, Andrew H, Neil W. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. https://doi.org/10.1056/NEJMoa0806470
11. Nicolucci A, Lucisano MScStat G, Vaccaro O et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol 2017;5:887–97. https://doi.org/10.1016/S2213-8587(17)30317-0
12. Rosak C, Mertes G. Critical evaluation of the role of acarbose in the treatment of diabetes: patient considerations. Diabetes Metab Syndr Obes 2012;5:357–67. https://doi.org/10.2147/DMSO.S28340
13. Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;(2):CD003639. https://doi.org/10.1002/14651858.CD003639.pub2
14. Bolen S, Wilson L, Vassy J et al. Comparative effectiveness and safety of oral diabetes medications for adults with type 2 diabetes. Agency for Healthcare Research and Quality (US); 2007. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20704051 [accessed 20 October 2017].
15. Chiasson J-L, Josse RG, Gomis R et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–7. https://doi.org/10.1016/S0140-6736(02)08905-5
16. Chiasson J-L, Josse RG, Gomis R et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance. JAMA 2003;290:486. https://doi.org/10.1001/jama.290.4.486
17. Holman RR, Coleman RL, Chan JCN et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2017;5:877–86. https://doi.org/10.1016/S2213-8587(17)30309-1
18. Holden SE, Gale EAM, Jenkins-Jones S, Currie CJ. How many people inject insulin? UK estimates from 1991 to 2010. Diabetes Obes Metab 2014;16:553–9. https://doi.org/10.1111/dom.12260
19. The ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–28. https://doi.org/10.1056/NEJMoa1203858
20. Mathieu C, Hollander P, Miranda-Palma B et al. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab 2013;98:1154–62. https://doi.org/10.1210/jc.2012-3249
21. Marso SP, McGuire DK, Zinman B et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med 2017;377:723–32. https://doi.org/10.1056/NEJMoa1615692
22. MacDonald MR, Eurich DT, Majumdar SR et al. Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested case-control study from the U.K. General Practice Research Database. Diabetes Care 2010;33:1213–18. https://doi.org/10.2337/dc09-2227
23. Ponikowski P, Voors AA, Anker SD et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–200. https://doi.org/10.1093/eurheartj/ehw128
24. Rojas LBA, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr 2013;5:6. https://doi.org/10.1186/1758-5996-5-6
25. Cleveland JC, Meldrum DR, Cain BS, Banerjee A, Harken AH. Oral sulfonylurea hypoglycemic agents prevent ischemic preconditioning in human myocardium. Two paradoxes revisited. Circulation 1997;96:29–32. https://doi.org/10.1161/01.CIR.96.1.29
26. Marx N, Rosenstock J, Kahn SE et al. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA(R)). Diabetes Vasc Dis Res 2015;12:164–174. https://doi.org/10.1177/1479164115570301
